Example alphapepttools workflow with proteomics data#
An example dataset: Alzheimer study#
This notebook demonstrates core alphapepttools functionality for proteomics data loading, preprocessing and visualization.
alphapepttools is designed to perform two main functions: First, provide a unified interface between search engine outputs and downstream processing. Second, provide downstream proteomics workflows entirely compatible with transcriptomics and the AnnData framework. Below we step through an alphapepttools example by using a published dataset by Bader et al. [2], who measured cerebrospinal fluid proteomes in order to discover biomarkers for Alzheimer’s disease.
[2]: Bader, Jakob M., et al. “Proteome profiling in cerebrospinal fluid reveals novel biomarkers of Alzheimer’s disease.” Molecular systems biology 16.6 (2020): e9356.
%load_ext autoreload
%autoreload 2
import tempfile
import numpy as np
import pandas as pd
import alphapepttools as at
# Data handling
from alphapepttools.io.anndata_factory import AnnDataFactory
from alphapepttools.pp.data import add_metadata, filter_by_metadata, filter_data_completeness
from alphapepttools.pp.transform import nanlog
from alphapepttools.pp.impute import impute_gaussian
from alphapepttools.tl.embeddings import pca
from alphapepttools.pp.batch_correction import scanpy_pycombat, drop_singleton_batches
from alphapepttools.pl.plots import Plots, add_lines, label_plot
from alphapepttools.pl.figure import create_figure, label_axes, save_figure
from alphapepttools.pl.colors import BaseColors, BasePalettes, BaseColormaps
Preparing the dataset using alphapepttools loaders and AnnData factory.#
In the case of this study, the full output of the DIANN search is saved as a report file of precursors, from which precursor or protein-level data can be extracted. alphapepttools handles this filtering with its AnnData factory class, drawing on the reader functionalities of alphabase. The resulting AnnData object contains protein-group quantities and any number of feature-metadata columns (for example, protein groups may have genes as secondary annotation, precursors may have protein groups and genes as secondary annotation).
# Download the dataset using the alphapepttools data module
output_dir = "./datasets/data_for_02_basic_analysis"
data_path = at.data.get_data("bader2020_full_diann", output_dir=output_dir if output_dir else tempfile.mkdtemp())
factory = AnnDataFactory.from_files(file_paths=data_path, reader_type="diann")
# Create the AnnData object, where the row index corresponds to samples and the column names correspond to proteins
adata = factory.create_anndata()
# Use the builtin dataframe conversion to get a quick overview of the data
display(adata.to_df().iloc[:5, :5])
./datasets/data_for_02_basic_analysis/report.parquet already exists (91.135817527771 MB)
| proteins | A0A075B6H7 | A0A075B6H9 | A0A075B6I0 | A0A075B6I1 | A0A075B6I9 |
|---|---|---|---|---|---|
| raw_name | |||||
| 20180618_QX0_JaBa_SA_LC12_5_CSF1_1_8-1xD1xS1fM1_sampleA01 | 515756544.0 | 37080368.0 | 10454921.0 | 5.520306e+06 | 36124044.0 |
| 20180618_QX0_JaBa_SA_LC12_5_CSF1_1_8-1xD1xS1fM1_sampleA02 | 433412480.0 | 35804032.0 | 45109720.0 | 4.065712e+06 | 45391408.0 |
| 20180618_QX0_JaBa_SA_LC12_5_CSF1_1_8-1xD1xS1fM1_sampleA03 | 262955984.0 | 32907378.0 | 10027142.0 | 1.697559e+06 | 29789766.0 |
| 20180618_QX0_JaBa_SA_LC12_5_CSF1_1_8-1xD1xS1fM1_sampleA04 | 286271648.0 | 19701684.0 | 11276591.0 | 1.086842e+07 | 40601508.0 |
| 20180618_QX0_JaBa_SA_LC12_5_CSF1_1_8-1xD1xS1fM1_sampleA05 | 447627040.0 | 50030340.0 | 51720148.0 | 8.910876e+06 | 117676152.0 |
Upstream data operation: log2 transformation#
when running alphapepttools.pp’s nanlog() We get a warning that our data contains NaN values, which are ignored in the log-transform.
When verbosity is set to 1, nanlog() alerts to special values for which log-transformation is not possible.
nanlog(adata, base=2, verbosity=1)
Integrating study metadata#
The AnnData format provides a solution to a key problem encountered in every data-analysis project: How to keep a matrix of numerical values permanently and safely aligned with column and row annotations. This is notably difficult with dataframes, as multilevel column indices are cumbersome and non-numeric columns in one dataframe cause problems with downstram analyses methods that expect numerical features. The add_metadata function from the preprocessing module ensures alignment of observations and variables from the start.
☝️ Importantly, while the original AnnData implementation only enforces shape compatibility, alphapepttools.pp.data.add_metadata() enforces matching indices. This means that even if the initial data and the incoming metadata were to be in different orders, quantitative and metadata information for a given sample are always matched.
# Download the metadata using the alphapepttools data module
output_dir = "./datasets/data_for_02_basic_analysis"
metadata_path = at.data.get_data("bader2020_metadata", output_dir=output_dir if output_dir else tempfile.mkdtemp())
metadata = pd.read_excel(metadata_path).dropna(subset=["sample name"])
./datasets/data_for_02_basic_analysis/annotation%20of%20samples_AM1.5.11.xlsx already exists (0.028104782104492188 MB)
# Basic cleaning of the metadata prior to merging
metadata["sample name"] = metadata["sample name"].str.replace(".raw.PG.Quantity", "", regex=False)
metadata = metadata.set_index("sample name", drop=False)
# The metadata contains information for more samples than are in our data
print(f"AnnData shape: {adata.shape}")
print(f"Metadata shape: {metadata.shape}")
AnnData shape: (61, 2162)
Metadata shape: (210, 14)
Since metadata and proteomics data have shared indices, matching is instant and safe:
# Match the metadata to the AnnData object
print(f"Anndata shape prior to matching: {adata.shape}")
adata = add_metadata(
adata=adata, # The AnnData object we want to add metadata to. Its obs index should match the index of the metadata
incoming_metadata=metadata, # The metadata dataframe we want to add. Its index should match the index of adata.obs
axis=0, # This means that we add metadata to the rows (0) and not columns (1)
keep_data_shape=False, # This means that we will drop any samples for which there is no corresponding row in the metadata
)
print(f"Anndata shape after matching: {adata.shape}")
print()
Anndata shape prior to matching: (61, 2162)
Anndata shape after matching: (61, 2162)
We can inspect that the metadata was correctly added:
# From now on, the metadata is stored in adata.obs
print("The sample-level metadata:")
display(adata.obs.head())
# For now, the feature (i.e. protein) metadata is a dataframe with only one column. We could easily add more protein annotations like GO-terms to it
print("The feature-level metadata:")
display(adata.var.head())
# And the protein abundances are stored in adata.X, which is a numpy array and perfectly suited for numerical operations
print("The protein abundances:")
display(adata.X[:5, :5])
The sample-level metadata:
The feature-level metadata:
The protein abundances:
| sample name | collection site | age | gender | t-tau [ng/L] | p-tau [ng/L] | Abeta-42 [ng/L] | Abeta-40 [ng/L] | Abeta ratio | biochemical AD classification | clinical AD diagnosis | MMSE score | cohort sample ID | comment | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| raw_name | ||||||||||||||
| 20180618_QX0_JaBa_SA_LC12_5_CSF1_1_8-1xD1xS1fM1_sampleA01 | 20180618_QX0_JaBa_SA_LC12_5_CSF1_1_8-1xD1xS1fM... | Sweden | 71.0 | f | 703.0 | 85.0 | 562.0 | NaN | NaN | biochemical control | NaN | NaN | ID_708 | NaN |
| 20180618_QX0_JaBa_SA_LC12_5_CSF1_1_8-1xD1xS1fM1_sampleA02 | 20180618_QX0_JaBa_SA_LC12_5_CSF1_1_8-1xD1xS1fM... | Sweden | 77.0 | m | 518.0 | 91.0 | 334.0 | NaN | NaN | biochemical AD | NaN | NaN | ID_722 | NaN |
| 20180618_QX0_JaBa_SA_LC12_5_CSF1_1_8-1xD1xS1fM1_sampleA03 | 20180618_QX0_JaBa_SA_LC12_5_CSF1_1_8-1xD1xS1fM... | Sweden | 75.0 | m | 974.0 | 87.0 | 515.0 | NaN | NaN | biochemical AD | NaN | NaN | ID_734 | NaN |
| 20180618_QX0_JaBa_SA_LC12_5_CSF1_1_8-1xD1xS1fM1_sampleA04 | 20180618_QX0_JaBa_SA_LC12_5_CSF1_1_8-1xD1xS1fM... | Sweden | 72.0 | f | 950.0 | 109.0 | 394.0 | NaN | NaN | biochemical AD | NaN | NaN | ID_742 | NaN |
| 20180618_QX0_JaBa_SA_LC12_5_CSF1_1_8-1xD1xS1fM1_sampleA05 | 20180618_QX0_JaBa_SA_LC12_5_CSF1_1_8-1xD1xS1fM... | Sweden | 63.0 | f | 873.0 | 88.0 | 234.0 | NaN | NaN | biochemical AD | NaN | NaN | ID_757 | NaN |
| proteins |
|---|
| A0A075B6H7 |
| A0A075B6H9 |
| A0A075B6I0 |
| A0A075B6I1 |
| A0A075B6I9 |
array([[28.942116, 25.144152, 23.317678, 22.396317, 25.106457],
[28.691166, 25.093618, 25.426935, 21.955076, 25.435915],
[27.970245, 24.971909, 23.257406, 20.69503 , 24.828314],
[28.09281 , 24.231815, 23.426828, 23.37364 , 25.27503 ],
[28.737722, 25.5763 , 25.624224, 23.087135, 26.810247]],
dtype=float32)
Use alphapepttools to generate an overview of the metadata#
In order to understand the properties of our data, we may want to generate overviews of certain columns in the metadata. To quickly generate publication-grade visuals, we utilize alphapepttools.pl.plots.Plots and its asssociated functionalities.
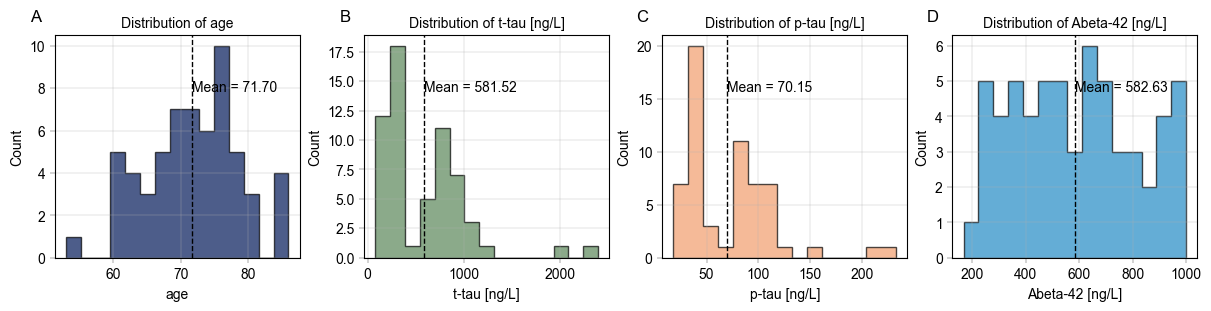
An example of create_figure’s iterative subplotting feature starts with specifying a number of numerical features we want to visualize:
# Check distribution of age and other numerical columns
numerical_columns = ["age", "t-tau [ng/L]", "p-tau [ng/L]", "Abeta-42 [ng/L]"]
☝️ A particular painpoint for many analyses is that the same levels should ideally have the same color throughout the entire analysis (i.e. it can be confusing if “disease” is colored red in one plot and green in the next). This can be annoyingly subtle to get right, which is why alphapepttools plots support a color_dict we can create upfront. This way, the same level is colored uniformly across all our plots.
# Assign a distinct color to each of our columns
palette = BasePalettes.get("qualitative", n=len(numerical_columns))
# Save the combination of numerical column and color in a dictionary
color_dict = dict(zip(numerical_columns, palette, strict=False))
Next, we instantiate a multi-panel figure with one facet for each of our numerical columns. A figure like this could be used for a supplementary figure, where the distribution of relevant columns should be shown.
fig, axm = create_figure(1, len(numerical_columns), figsize=(3 * len(numerical_columns), 3))
# Iterate over the columns and generate the histograms.
# Note that we also iterate over a string of letters to label the individual subplots with A, B, C, ...
for col, enumeration in zip(numerical_columns, "ABCDEFGHIJKLMNOP", strict=False):
ax = axm.next() # Go to the next facette of the AxesManager
# Plot the histogram for the current column
Plots.histogram(
ax=ax,
bins=15,
data=adata,
value_column=col,
color=color_dict[col],
# Add whatever flourish we want for the histograms
hist_kwargs={
"histtype": "stepfilled",
"alpha": 0.7,
"edgecolor": "black",
},
)
# Label the individual axes (note that this takes the Axes object as an argument, so
# everything gets layered onto the same figure)
label_axes(
ax=ax,
xlabel=col,
ylabel="Count",
title=f"Distribution of {col}",
enumeration=enumeration,
)
# Indicate means with a vertical line
mean = adata.obs[col].mean() # calculate the mean of the current column in the metadata
add_lines(
ax=ax,
intercepts=mean,
linetype="vline",
color="black",
)
# Add a label for each mean
label_plot(
ax=ax,
x_values=[mean],
y_values=[0.75],
labels=[f"Mean = {mean:.2f}"],
# The code is necessary to position all mean labels at the same height.
# It essentially means "Use data coordinates for the x-axis, but use axes coordinates (0-1) for the y-axis".
label_kwargs={"transform": ax.get_xaxis_transform()},
)
# Since the figure size is fixed from the initial generation of the subplot, we can save it looking exactly as we see below in the notebook
save_figure(
fig=fig, # Note that here we're using the whole figure to whose axes we added our plots
filename="continuous_var_overview.svg",
output_dir="./example_outputs",
)
# TODO: Implement barplots for categorical variables.
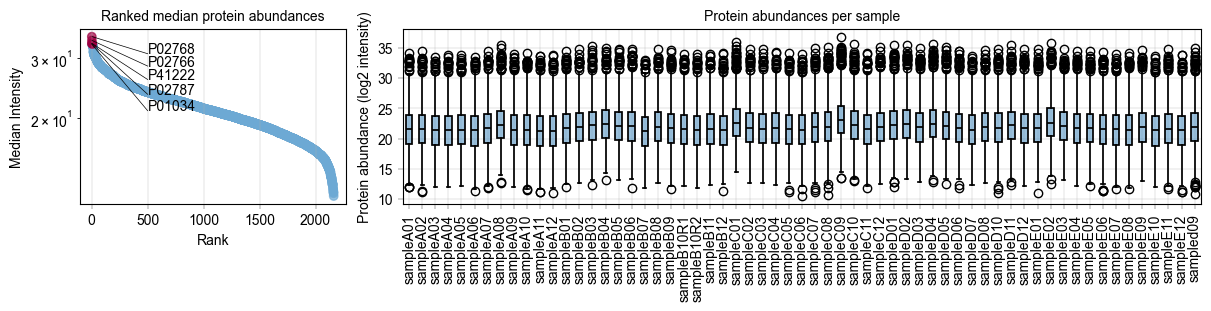
Next,#
we would like a small panel to highlight the distribution of our protein intensities with highlights the 10 most abundant proteins, and look at the protein distribution across all our samples to check if we need imputation.
# Some custom code to find the 5 most abundant proteins
protein_abundances = np.nanmedian(adata.X, axis=0)
top_5_protein_indices = np.argsort(protein_abundances)[-5:]
top_5_protein_names = adata.var_names[top_5_protein_indices]
top_5_protein_medians = protein_abundances[top_5_protein_indices]
# Sort the top 5 proteins by their median abundance
median_order = np.argsort(top_5_protein_medians)
top_5_protein_medians = top_5_protein_medians[median_order]
top_5_protein_names = top_5_protein_names[median_order]
# Mark the top proteins in the anndata object
adata.var["status"] = "other"
adata.var.loc[top_5_protein_names, "status"] = "top 5"
# Specify how to color the ranked proteins
rank_color_dict = {"top 5": BaseColors.get("red"), "other": BaseColors.get("lightblue")}
# Generate a panel to contain the rank plot and the protein abundance boxplots
fig, axm = create_figure(1, 2, figsize=(12, 3), gridspec_kwargs={"width_ratios": [1, 3]})
# First, show the protein values
ax = axm.next()
Plots.rank_median_plot(
data=adata,
ax=ax,
color_map_column="status", # The column to indicate which proteins are special
color_dict=rank_color_dict, # How to color the special proteins
scatter_kwargs={
"alpha": 0.7,
},
)
label_axes(
ax=ax,
title="Ranked median protein abundances",
)
label_plot(
ax=ax,
x_values=range(len(top_5_protein_names)),
y_values=top_5_protein_medians[median_order],
labels=top_5_protein_names[median_order],
x_anchors=[500],
y_padding_factor=4.5,
)
# Next, show the protein intensity per sample
ax = axm.next()
Plots.boxplot(
ax=ax,
data=adata.T,
direct_columns=adata.obs.index.tolist(),
)
# This is a critical strength of not having a custom end-to-end plotting function:
# The ax object is always accessible, so a little matplotlib code can get rid of
# cumbersome long xtick labels:
xtick_labels = [item.get_text() for item in ax.get_xticklabels()] # Get xtick labels directly from axes
xtick_labels = [x.split("_")[-1] for x in xtick_labels] # Shorten the xtick labels to make them more readable
_ = ax.set_xticklabels(xtick_labels, rotation=90) # Set the new xtick labels
label_axes(
ax=ax,
title="Protein abundances per sample",
ylabel="Protein abundance (log2 intensity)",
)
# Save the figure
save_figure(
fig=fig, # Note that here we're using the whole figure to whose axes we added our plots
filename="protein_abundance_overview.svg",
output_dir="./example_outputs",
)
Filter study data based on metadata#
In order to analyse subcohorts of the study, it can be useful to filter by a metadata variable like age or gender. alphapepttools offers an intuitive way to do so via alphapepttools.pp.data.filter_by_metadata(), which takes a dictionary of filter conditions returns a subsetted AnnData object.
# Example: Subset to samples where 'gender' is "f":
adata_f = filter_by_metadata(
adata=adata,
filter_dict={"gender": "f"},
axis=0, # samples
action="keep",
)
print(adata_f.obs["gender"].value_counts())
# Example: Subset to samples where 'gender' is "f" and 'age' is over 70:
adata_f_over70 = filter_by_metadata(
adata=adata,
filter_dict={
"gender": "f",
"age": (70, None), # None means no upper bound
},
axis=0, # samples
logic="and", # Both conditions have to be met
action="keep",
)
print(adata_f_over70.obs["gender"].value_counts())
print(adata_f_over70.obs["age"].describe())
# Example:
gender
f 24
Name: count, dtype: int64
gender
f 16
Name: count, dtype: int64
count 16.000000
mean 73.312500
std 3.198307
min 70.000000
25% 71.000000
50% 72.000000
75% 74.250000
max 80.000000
Name: age, dtype: float64
Filter proteins based on their completeness across samples#
A frequent action is to get the “core proteome” of a dataset, i.e. the proteins that have no missing values in any sample. We can perform this via our filter_data_completeness function by setting the max_missing argument to 0.
print(f"Number of proteins in the study: {adata.X.shape[1]}")
adata_core = filter_data_completeness(
adata=adata,
max_missing=0,
action="drop",
)
print(f"Number of proteins with complete data: {adata_core.X.shape[1]}")
if adata_core.to_df().isna().sum().sum() != 0:
raise ValueError("There are still missing values in the data after filtering for complete proteins.")
else:
print("No missing values remain after filtering for complete proteins.")
Number of proteins in the study: 2162
Number of proteins with complete data: 1136
No missing values remain after filtering for complete proteins.
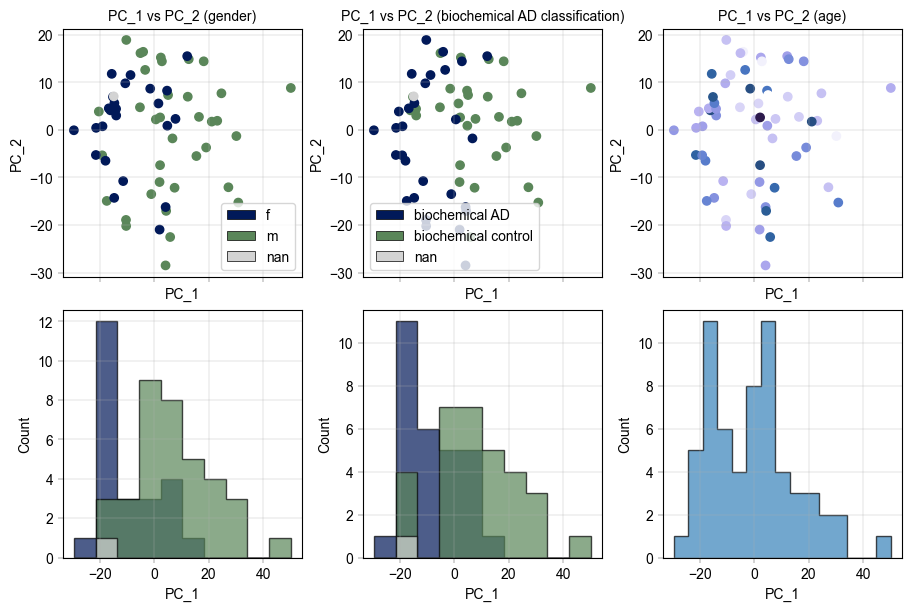
Examine the PCA clustering of data, colored by different variables#
PCA values are computed and saved to the same AnnData instance, making use of its obsm, varm, and uns (unstructured) data fields.
# We restrict our dataset to proteins with no more than 25 % missing values across all samples
adata_25pc = adata.copy()
filter_data_completeness(
adata=adata_25pc,
max_missing=0.25,
action="drop",
)
# We impute the remaining missing values from a downshifted gaussian distribution
impute_gaussian(
adata=adata_25pc,
)
# Add PCA embeddings to the AnnData object by utilizing its 'obsm' attribute
pca(adata_25pc, n_comps=10)
# Locating the PCA results
print("\nPCA Components: adata.obsm['X_pca'] with shape (n_obs x n_comps):")
print(adata_25pc.obsm["X_pca_obs"].shape)
print("\nPCA loadings: adata.varm['PCs'] with shape (n_var x n_comps):")
print(adata_25pc.varm["PCs_obs"].shape)
print("\nRatio of explained variance: uns['pca']['variance_ratio'] with shape (n_comps,):")
print(adata_25pc.uns["variance_pca_obs"]["variance_ratio"])
print("\nExplained variance: uns['pca']['variance'] with shape (n_comps):")
print(adata_25pc.uns["variance_pca_obs"]["variance"])
PCA Components: adata.obsm['X_pca'] with shape (n_obs x n_comps):
(61, 10)
PCA loadings: adata.varm['PCs'] with shape (n_var x n_comps):
(2162, 10)
Ratio of explained variance: uns['pca']['variance_ratio'] with shape (n_comps,):
[0.16833097 0.08768225 0.04490079 0.03995479 0.03662875 0.03373726
0.0291991 0.02595061 0.02371496 0.02261774]
Explained variance: uns['pca']['variance'] with shape (n_comps):
[251.18832 130.842 67.00225 59.621685 54.65847 50.343723
43.571735 38.724243 35.38814 33.75085 ]
# Visualizing the PCA results
fig, axm = create_figure(2, 3, figsize=(9, 6), subplots_kwargs={"sharex": True})
# We can get the PCA component values into a dataframe
pca_df = pd.DataFrame(
adata_25pc.obsm["X_pca_obs"],
index=adata_25pc.obs.index,
columns=[f"PC_{i + 1}" for i in range(adata_25pc.obsm["X_pca_obs"].shape[1])],
)
pca_df = pca_df.join(adata_25pc.obs)
# Color by gender
ax = axm.next()
Plots.scatter(
ax=ax,
data=pca_df,
x_column="PC_1",
y_column="PC_2",
color_map_column="gender",
legend="auto",
)
label_axes(
ax=ax,
xlabel="PC_1",
ylabel="PC_2",
title="PC_1 vs PC_2 (gender)",
)
# TODO: Scatter should have an additional x_labels and y_labels argument, making it possible to pass ranking information to x_values and a_values but display categorical levels of interest.
# Color by biochemical AD classification
ax = axm.next()
Plots.scatter(
ax=ax,
data=pca_df,
x_column="PC_1",
y_column="PC_2",
color_map_column="biochemical AD classification",
legend="auto",
)
label_axes(
ax=ax,
xlabel="PC_1",
ylabel="PC_2",
title="PC_1 vs PC_2 (biochemical AD classification)",
)
# Color by age
# TODO: Legend with small colorbar when a colormap is used
ax = axm.next()
Plots.scatter(
ax=ax,
data=pca_df,
x_column="PC_1",
y_column="PC_2",
color_map_column="age",
palette=BaseColormaps.get("sequential"),
)
label_axes(
ax=ax,
xlabel="PC_1",
ylabel="PC_2",
title="PC_1 vs PC_2 (age)",
)
# TODO: add_labels should work on anndata directly
# Go on and add histograms for first component
ax = axm.next()
Plots.histogram(
ax=ax,
data=pca_df,
value_column="PC_1",
color_map_column="gender",
hist_kwargs={
"histtype": "stepfilled",
"alpha": 0.7,
"edgecolor": "black",
},
)
label_axes(
ax=ax,
xlabel="PC_1",
ylabel="Count",
)
ax = axm.next()
Plots.histogram(
ax=ax,
data=pca_df,
value_column="PC_1",
color_map_column="biochemical AD classification",
hist_kwargs={
"histtype": "stepfilled",
"alpha": 0.7,
"edgecolor": "black",
},
)
label_axes(
ax=ax,
xlabel="PC_1",
ylabel="Count",
)
ax = axm.next()
Plots.histogram(
ax=ax,
data=pca_df,
bins=15,
value_column="PC_1",
hist_kwargs={
"histtype": "stepfilled",
"alpha": 0.7,
"edgecolor": "black",
},
)
label_axes(
ax=ax,
xlabel="PC_1",
ylabel="Count",
)
Or a more creative approach to one of the variables, e.g. Alzheimer’s classification:#
# Visualizing the PCA results
fig, axm = create_figure(2, 2, figsize=(6, 6))
# We can get the PCA component values into a dataframe
pca_df = pd.DataFrame(
adata_25pc.obsm["X_pca_obs"],
index=adata_25pc.obs.index,
columns=[f"PC_{i + 1}" for i in range(adata_25pc.obsm["X_pca_obs"].shape[1])],
)
pca_df = pca_df.join(adata_25pc.obs)
# Ensure that the colors are the same across all plots
color_dict = {
"biochemical control": BaseColors.get("blue"),
"biochemical AD": BaseColors.get("red"),
"NA": BaseColors.get("grey"),
}
# Color by biochemical AD classification
ax = axm.next()
Plots.scatter(
ax=ax,
data=pca_df,
x_column="PC_1",
y_column="PC_2",
color_map_column="biochemical AD classification",
legend="auto",
color_dict=color_dict,
)
label_axes(
ax=ax,
xlabel="PC_1",
ylabel="PC_2",
title="PC_1 vs PC_2 (biochemical AD classification)",
)
# Move the y-axis label to the right side of the plot
ax.yaxis.set_label_position("right")
ax.yaxis.tick_right()
# Go on and add 90 degree tilted histograms for the second component
ax = axm.next()
Plots.histogram(
ax=ax,
data=pca_df,
value_column="PC_2",
color_map_column="biochemical AD classification",
hist_kwargs={
"histtype": "stepfilled",
"alpha": 0.7,
"edgecolor": "black",
"orientation": "horizontal",
},
color_dict=color_dict,
)
label_axes(
ax=ax,
xlabel="Count",
)
ax.xaxis.set_label_position("top")
# Add a histogram for the first component
ax = axm.next()
Plots.histogram(
ax=ax,
data=pca_df,
value_column="PC_1",
color_map_column="biochemical AD classification",
hist_kwargs={
"histtype": "stepfilled",
"alpha": 0.7,
"edgecolor": "black",
},
color_dict=color_dict,
)
label_axes(
ax=ax,
ylabel="Count",
)
ax.invert_yaxis()
# And lastly a scree plot to show the explained varince
ax = axm.next()
Plots.scree_plot(
ax=ax,
adata=adata_25pc,
n_pcs=10,
color=BaseColors.get("green"),
)
label_axes(
ax=ax,
xlabel="Principal component",
ylabel="Explained variance",
)
ax.xaxis.set_label_position("top")
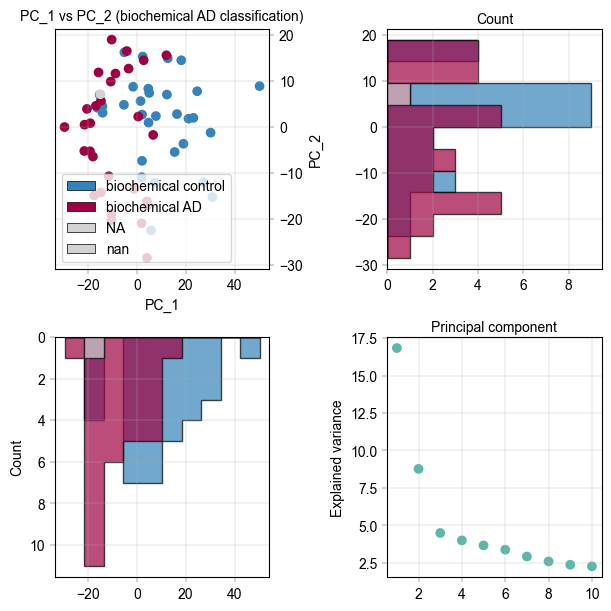
It appears there is separation of the Alzheimer’s and control samples on the first principal component. We can examine the loadings of the first component to get an idea of which proteins are regulated.
fig, axm = create_figure(1, 1, figsize=(6, 6))
# TODO: All plots should have an optional ax argument, e.g. if no ax is given, they should call create_figure(1,1) internally
Plots.plot_pca_loadings(
data=adata_25pc,
ax=axm.next(),
dim=1,
)
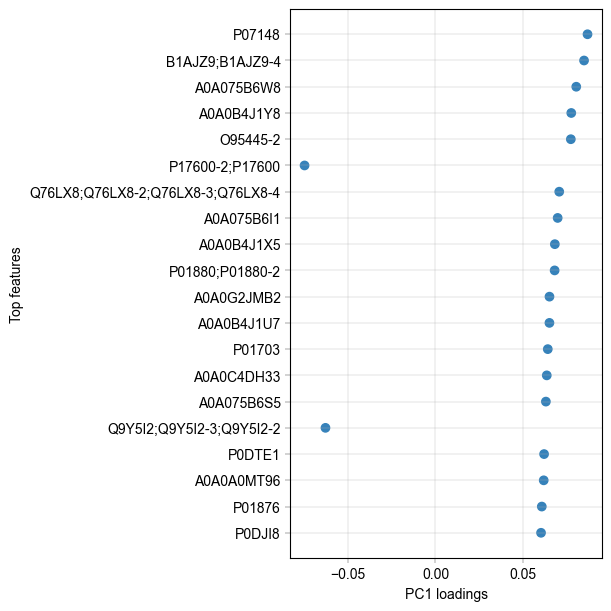
The two highest loadings on PC1 are LV949 and A0A075B6W8. We can take these along and check their differential abundance between treatments.
Batch correction#
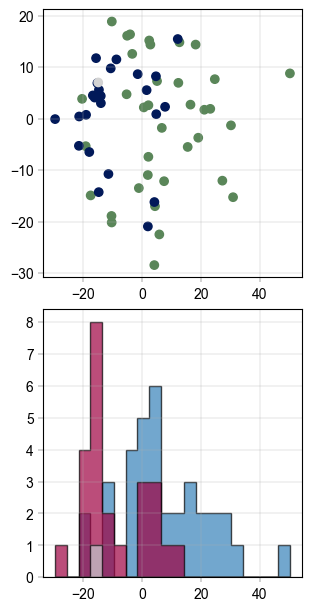
A simple and effective way of correcting for batch effects was published under the name ComBat [REF]. Based on Empirical Bayes methods, it can remove technical or sampling batch effects while retaining biological information.
The function implementation has two major failure modes which causes batch correction to fail: A) If a batch occurs exactly once or B) if there are NaN-values in the data. We can check for both and mitigate them:
impute_gaussian(adata_25pc)
adata_25pc = drop_singleton_batches(adata_25pc, batch="gender")
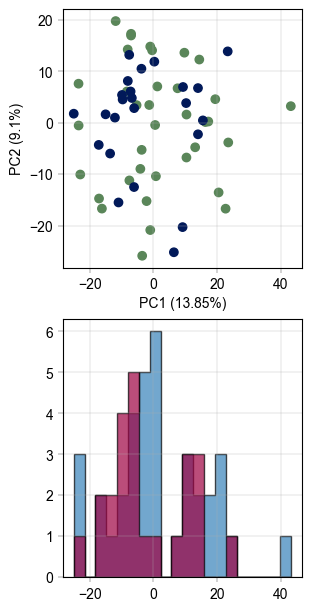
# visualize the data before batch correction
fig, axm = create_figure(2, 1, figsize=(3, 6))
Plots.scatter(
ax=axm.next(),
data=pca_df,
x_column="PC_1",
y_column="PC_2",
color_map_column="gender",
)
Plots.histogram(
ax=axm.next(),
data=pca_df,
value_column="PC_1",
bins=20,
color_map_column="gender",
hist_kwargs={
"histtype": "stepfilled",
"alpha": 0.7,
"edgecolor": "black",
},
color_dict={
"m": BaseColors.get("blue"),
"f": BaseColors.get("red"),
},
)
# Apply PyCombat batch correction
adata_25pc_corr = adata_25pc.copy()
scanpy_pycombat(
adata=adata_25pc_corr,
batch="gender",
)
Found 7 genes with zero variance.
# recompute pca TODO: Remove dataframe based plotting for pca_plot or adapter down the line
pca(adata_25pc_corr, n_comps=10)
pca_df_corr = pd.DataFrame(
adata_25pc_corr.obsm["X_pca_obs"],
index=adata_25pc_corr.obs.index,
columns=[f"PC_{i + 1}" for i in range(adata_25pc_corr.obsm["X_pca_obs"].shape[1])],
)
pca_df_corr = pca_df_corr.join(adata_25pc_corr.obs)
# Visualize again
fig, axm = create_figure(2, 1, figsize=(3, 6))
Plots.plot_pca(
ax=axm.next(),
data=adata_25pc_corr,
x_column=1,
y_column=2,
color_map_column="gender",
)
Plots.histogram(
ax=axm.next(),
data=pca_df_corr,
value_column="PC_1",
bins=20,
color_map_column="gender",
hist_kwargs={
"histtype": "stepfilled",
"alpha": 0.7,
"edgecolor": "black",
},
color_dict={
"m": BaseColors.get("blue"),
"f": BaseColors.get("red"),
},
)