A Walk Through The scPortrait Ecosystem#
This notebook will introduce you to the scPortrait ecosystem and give you a complete working example of how to use scPortrait. It will walk you through the following steps of the scPortrait workflow:
Segmentation: Generates masks for the segmentation of input images into individual cells
Extraction: The segmentation masks are applied to extract single-cell images for all cells in the input images. Images of individual cells are rescaled to [0, 1] per channel.
Featurization: The image-based phenotype of each individual cell in the extracted single-cell dataset is featurized using the specified featurization method. Multiple featurization runs can be performed on the same dataset using different methods. Here we utilize the pretrained binary classifier from the original SPARCS manuscript that identifies individual cells defective in a biological process called “autophagy”.
Selection: Cutting instructions for the isolation of selected individual cells by laser microdissection are generated. The cutting shapes are written to an
.xmlfile which can be loaded on a Leica LMD7 microscope for automated cell excision.
The data used in this notebook was previously stitched using the stitching workflow in SPARCStools. Please see the notebook here.
Import Required Libraries#
First we need to import all of the python functions we require to run the pipeline.
[1]:
import os
import matplotlib.pyplot as plt
import numpy as np
from scportrait.pipeline.featurization import MLClusterClassifier
from scportrait.pipeline.extraction import HDF5CellExtraction
from scportrait.pipeline.project import Project
from scportrait.pipeline.segmentation.workflows import WGASegmentation
from scportrait.pipeline.selection import LMDSelection
from scportrait.data._datasets import dataset_1 # to download example dataset
import scportrait
import yaml
from IPython.display import Markdown
Generate the Project Structure#
Executing this code will generate a new scPortrait project in the designated location. Each scPortrait project has the following general structure:
.
├── classification
│ └── classifier_name
│ └── processing.log
├── config.yml
├── extraction
├── segmentation
└── processing.log
[2]:
project_location = "../example_projects/example_1/project_walkthrough"
config_file = scportrait.data.get_config_file(config_id = "dataset_1_wga_config")
project = Project(
os.path.abspath(project_location),
config_path=config_file,
overwrite=True,
debug=True,
segmentation_f=WGASegmentation,
extraction_f=HDF5CellExtraction,
featurization_f=MLClusterClassifier,
selection_f=LMDSelection,
)
Updating project config file.
[10/04/2025 21:47:43] Loading config from /Users/sophia/Documents/GitHub/scPortrait/examples/notebooks/example_projects/example_1/project_walkthrough/config.yml
[10/04/2025 21:47:44] No cache directory specified in config using current working directory /Users/sophia/Documents/GitHub/scPortrait/examples/notebooks/tutorials.
[10/04/2025 21:47:44] Compression algorithm for extracted single-cell images: lzf
[10/04/2025 21:47:44] No cache directory specified in config using current working directory /Users/sophia/Documents/GitHub/scPortrait/examples/notebooks/tutorials.
Load Imaging Data#
Once we have generated our project structure we next need to load our imaging data. There are several different ways to do this.
you can load the images directly from file by specifying a list of filepaths
you can load the images from numpy arrays that are already loaded into memory
Here it is important that you load the channels in the following order: Nucleus, Cellmembrane, others
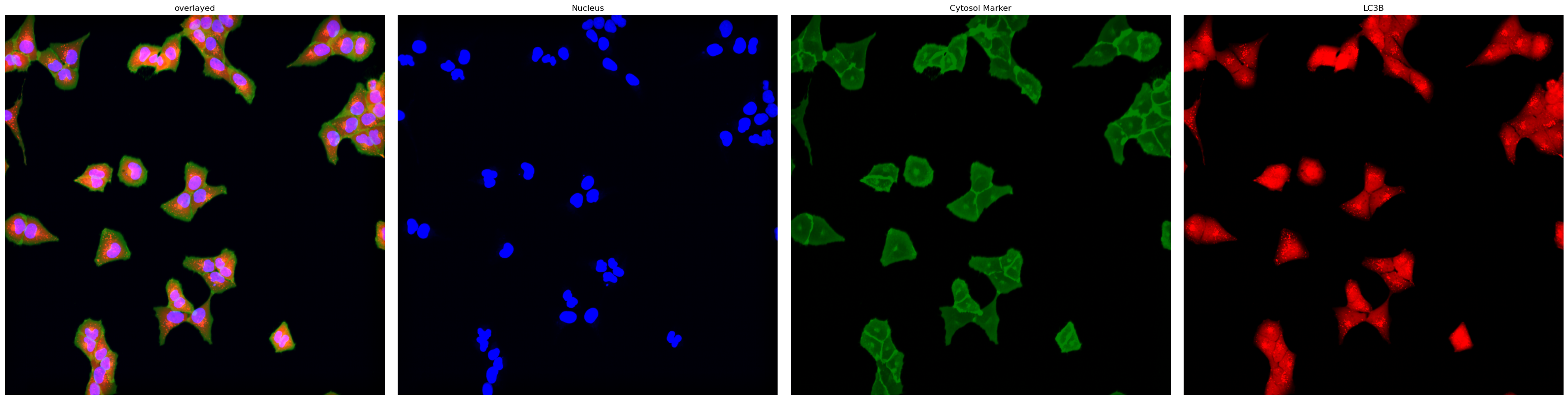
In this particular example we are utilizing images from U2OS cells which are stained with Hoechst 33342 to visualize nuclei and express Lck-mNeon to visualize the cellular membrane and LC3B-mcherry, a fluroescently tagged protein relevant for visualizing the biological process of autophagy. The cells have not been stimulated to induce autophagy and should be autophagy defective.
Method 1: loading images from file#
[3]:
path = dataset_1()
images = [
f"{path}/Ch1.tif",
f"{path}/Ch2.tif",
f"{path}/Ch3.tif",
]
project.load_input_from_tif_files(images, channel_names=["Nucleus", "Cytosol Marker", "LC3B"])
[10/04/2025 21:47:44] Output location /Users/sophia/Documents/GitHub/scPortrait/examples/notebooks/example_projects/example_1/project_walkthrough/scportrait.sdata already exists. Overwriting.
INFO The Zarr backing store has been changed from None the new file path:
/Users/sophia/Documents/GitHub/scPortrait/examples/notebooks/example_projects/example_1/project_walkthroug
h/scportrait.sdata
[10/04/2025 21:47:44] Initialized temporary directory at /Users/sophia/Documents/GitHub/scPortrait/examples/notebooks/tutorials/Project_xwum9g1r for Project
[10/04/2025 21:47:44] Image input_image written to sdata object.
[10/04/2025 21:47:44] Cleaned up temporary directory at <TemporaryDirectory '/Users/sophia/Documents/GitHub/scPortrait/examples/notebooks/tutorials/Project_xwum9g1r'>
Method 2: loading images from numpy array#
To simulate the case where the images you want to load are already loaded as a numpy array, we first convert the images to a numpy array and then pass this array to the project instead of only providing the image paths.
[4]:
from tifffile import imread
path = dataset_1()
images = [
f"{path}/Ch1.tif",
f"{path}/Ch2.tif",
f"{path}/Ch3.tif",
]
image_arrays = np.array([imread(path) for path in images])
project.load_input_from_array(image_arrays, channel_names=["Nucleus", "Cytosol Marker", "LC3B"])
[10/04/2025 21:47:45] Output location /Users/sophia/Documents/GitHub/scPortrait/examples/notebooks/example_projects/example_1/project_walkthrough/scportrait.sdata already exists. Overwriting.
INFO The Zarr backing store has been changed from None the new file path:
/Users/sophia/Documents/GitHub/scPortrait/examples/notebooks/example_projects/example_1/project_walkthroug
h/scportrait.sdata
[10/04/2025 21:47:45] Image input_image written to sdata object.
Looking at the loaded images#
The loaded images are accesible via the input_image parameter of the project. They are saved into the underlying spatialdata object.
[5]:
project.input_image
[5]:
<xarray.DataArray 'image' (c: 3, y: 3040, x: 3038)> Size: 55MB
dask.array<from-zarr, shape=(3, 3040, 3038), dtype=uint16, chunksize=(3, 256, 256), chunktype=numpy.ndarray>
Coordinates:
* c (c) <U14 168B 'Nucleus' 'Cytosol Marker' 'LC3B'
* y (y) float64 24kB 0.5 1.5 2.5 3.5 ... 3.038e+03 3.038e+03 3.04e+03
* x (x) float64 24kB 0.5 1.5 2.5 3.5 ... 3.036e+03 3.036e+03 3.038e+03
Attributes:
transform: {'global': Identity }You can easily visualize a center cropped image excerpt using the .plot_input_image() helper function.
[6]:
project.plot_input_image()
Alternatively, you can also visualize the input images as well as all other objects saved in a spatialdata object in an interactive napari viewer. This will only work if running locally.
[7]:
project.view_sdata()
After finishing with the interactive viewing you can close the viewer with .close_interactive_viewer(). This will also write any additional elements created back to disk.
[8]:
project.close_interactive_viewer()
Writing the following manually added files to the sdata object: []
2025-04-10 21:47:48.413 python[95616:3747651] +[IMKClient subclass]: chose IMKClient_Modern
2025-04-10 21:47:48.413 python[95616:3747651] +[IMKInputSession subclass]: chose IMKInputSession_Modern
Generating a Segmentation#
scPortrait has different segmentation workflows between which you can choose. If none of them fit your needs you can also easily write your own.
Here we will demonstrate the CPU based classical segmentation method that was also utilized in the manuscript.
We define the segmentation method to be used when we initialize the project. In this case we used the WGASegmentation method. The ShardedWGASegmentation works very similarily but allows you to process several image chunks in parallel for more efficient computation on large input images.
By specifying debug = True we can see intermediate output results from the segmentation.
The WGASegmentation method relies on parameters specified in the config.yml we loaded when initializing our project.
[9]:
display(Markdown(f"```yaml\n{yaml.dump(project.segmentation_f.config, default_flow_style=False)}\n```"))
cache: /Users/sophia/Documents/GitHub/scPortrait/examples/notebooks/tutorials
chunk_size: 50
cytosol_segmentation:
dilation: 7
erosion: 2
lower_quantile_normalization: 0.01
max_clip: 0.2
max_size: 6000
min_clip: 0
min_size: 200
threshold: 0.05
upper_quantile_normalization: 0.99
filter_masks_size: true
input_channels: 3
lower_quantile_normalization: 0.001
median_filter_size: 4
nucleus_segmentation:
contact_filter: 0.5
dilation: 0
lower_quantile_normalization: 0.01
max_size: 1000
median_block: 41
median_step: 4
min_distance: 8
min_size: 200
peak_footprint: 7
speckle_kernel: 9
threshold: 0.2
upper_quantile_normalization: 0.99
upper_quantile_normalization: 0.999
By passing the parameter debug = True we tell scPortrait to also generate intermediate outputs which we can look at to see the different segmentation steps. It is only recommended to do this for debugging or visualization purposes as it will utilize more memory and be slower.
For the WGASegmentation method the intermediate outputs that are displayed are the following:
percentile normalized input images for each of the three channels (3 images)
median normalized input images (this slightl smooths the images for a better segmentation result) (3 images)
histogram showing the intensity distribution for nuclear and cytosolic channel (2 plots)
generated mask after applying nuclear thresholding
nuclear thresholding mask with calculated centers for each detected nucleus
fast marching map with nuclei centers indicated in red
segmented individual nuclei (2 different visualizations)
starting nucleus mask for filtering
histrogram showing size distribution of segmented nuclei
segmentation mask with too small nuclei shown in red
segmentation mask with too large nuclei shown in red
WGA thresholding mask
WGA potential map
WGA fast marching results
Watershed results with nuclear centers shown in red
WGA mask
Cytosol size distribution
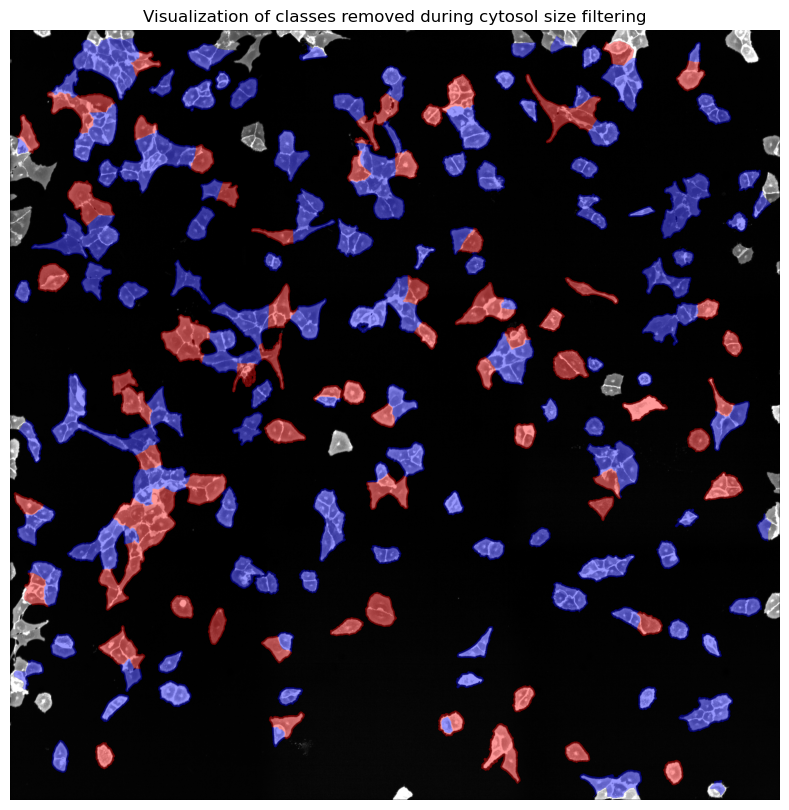
cytosol mask with too small cells shown in red
cytosol mask with too large cells shown in red
[10]:
project.segment()
[10/04/2025 21:47:49] Normalization of the input image will be performed.
[10/04/2025 21:47:49] Normalizing each channel to the same range
[10/04/2025 21:47:50] Performing median filtering on input image
[10/04/2025 21:48:00] Percentile normalization of nucleus input image to range 0.01, 0.99
[10/04/2025 21:48:00] Normalizing each channel to the same range
[10/04/2025 21:48:00] Using local thresholding with a threshold of 0.2 to calculate nucleus mask.
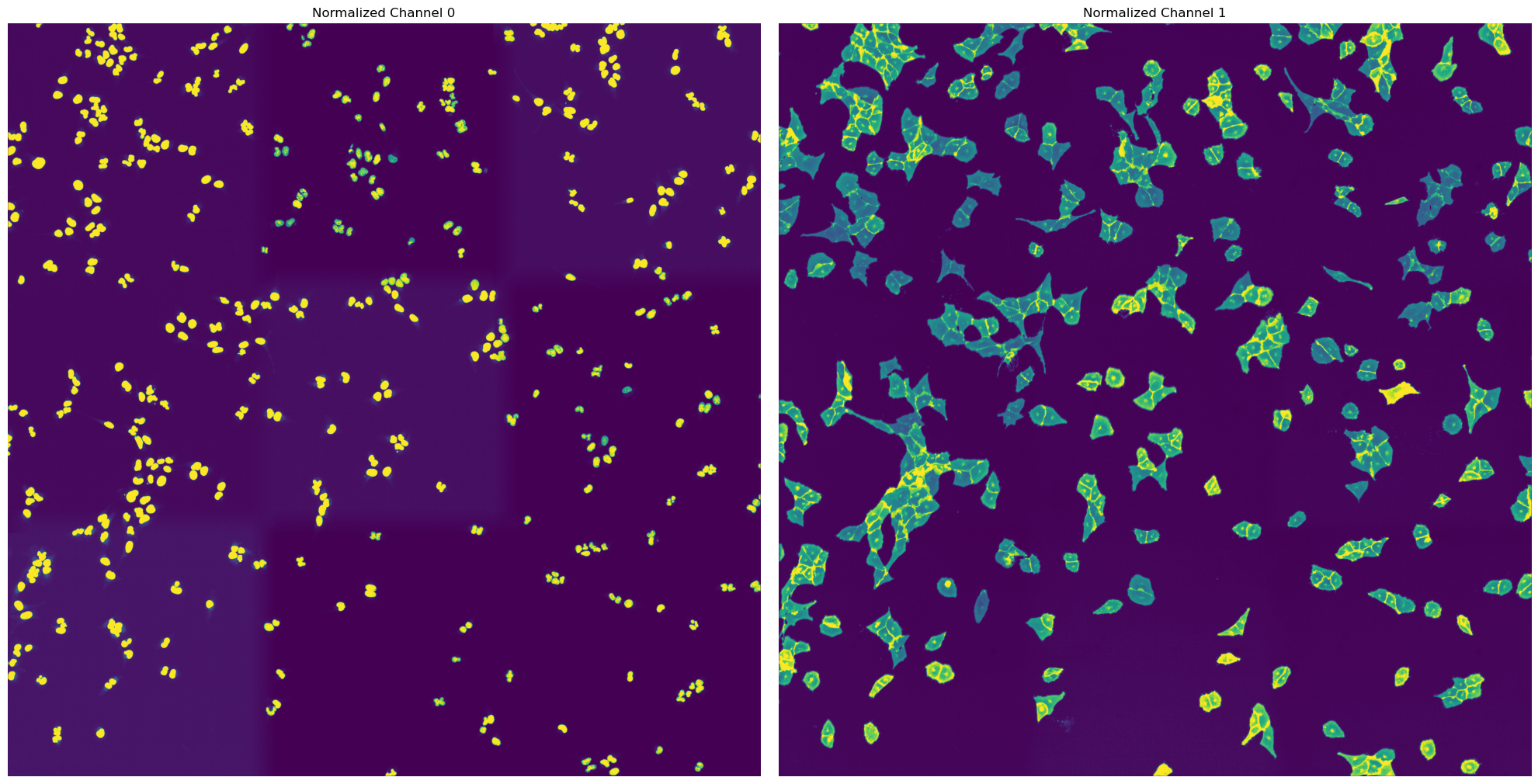
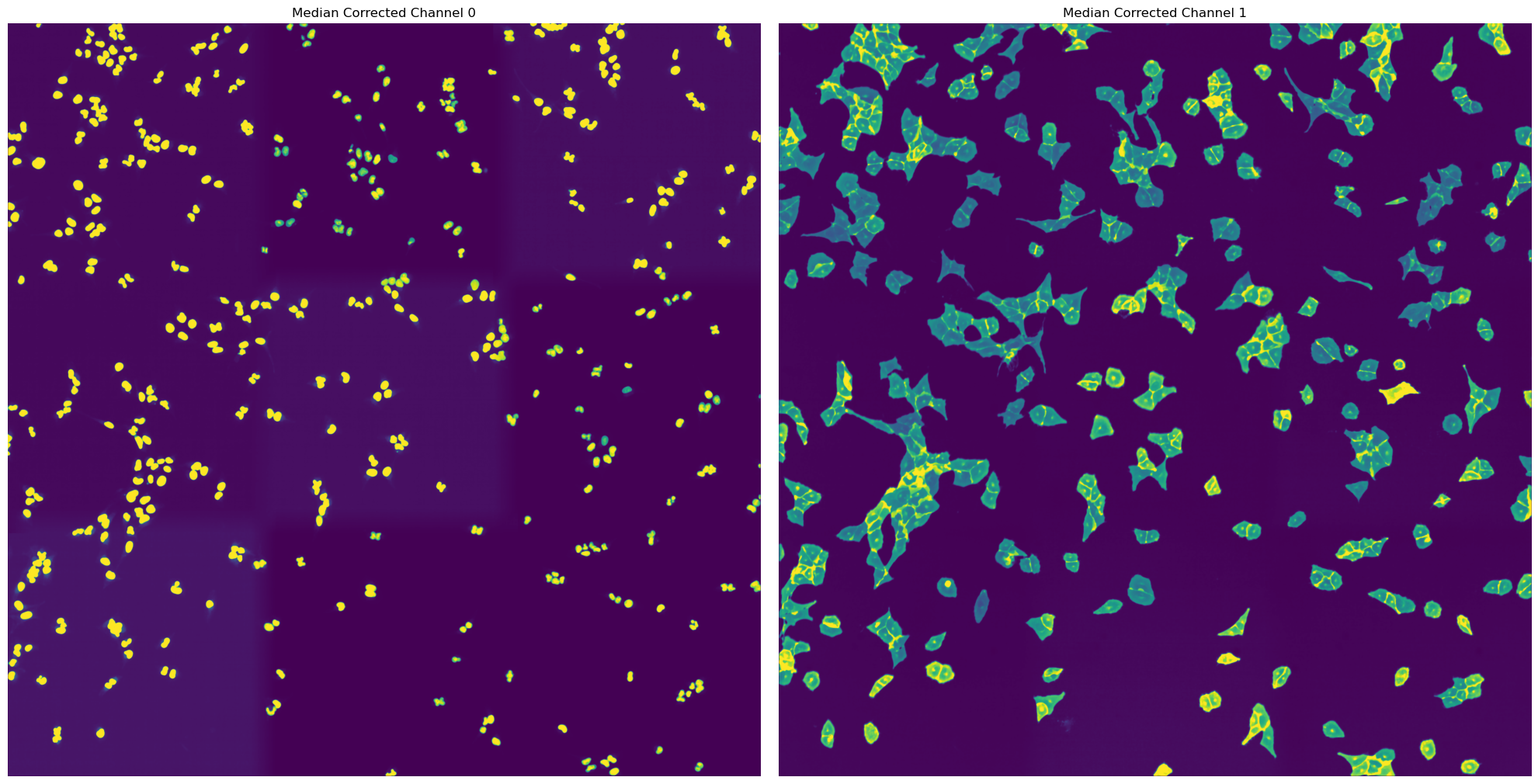


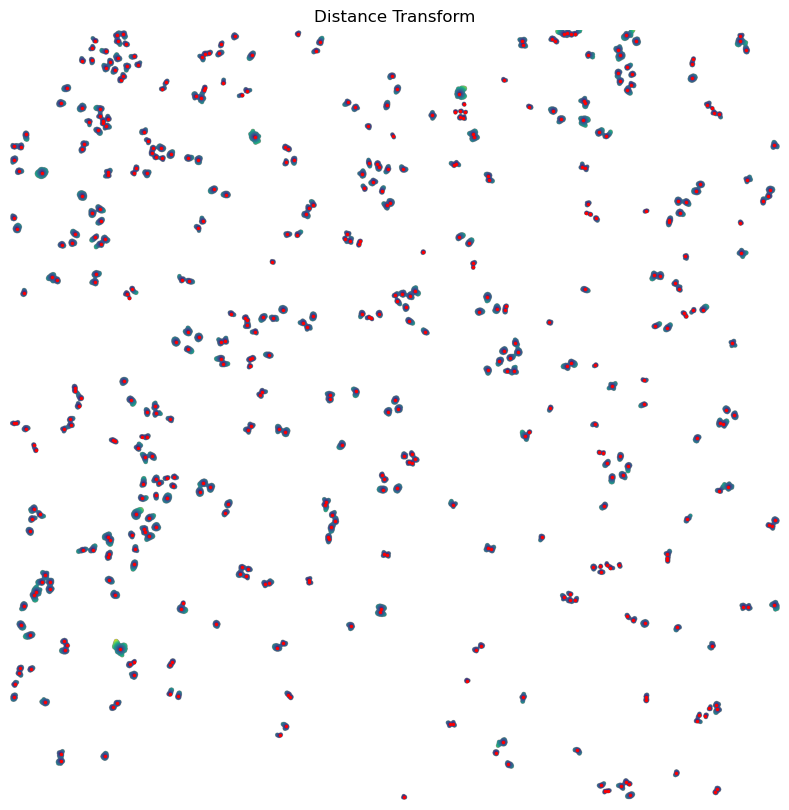
[10/04/2025 21:48:17] Performing filtering of nucleus with specified thresholds [200, 1000] from config file.
[10/04/2025 21:48:17] Removed 47 nuclei as they fell outside of the threshold range [200, 1000].
[10/04/2025 21:48:18] Total time to perform nucleus size filtering: 1.0620636940002441 seconds
OMP: Info #276: omp_set_nested routine deprecated, please use omp_set_max_active_levels instead.
[10/04/2025 21:48:19] Filtered out 6 nuclei due to contact filtering.
[10/04/2025 21:48:19] Normalizing each channel to the same range
[10/04/2025 21:48:22] Cytosol Potential Mask generated.
[10/04/2025 21:48:24] Performing filtering of cytosol with specified thresholds [200, 6000] from config file.
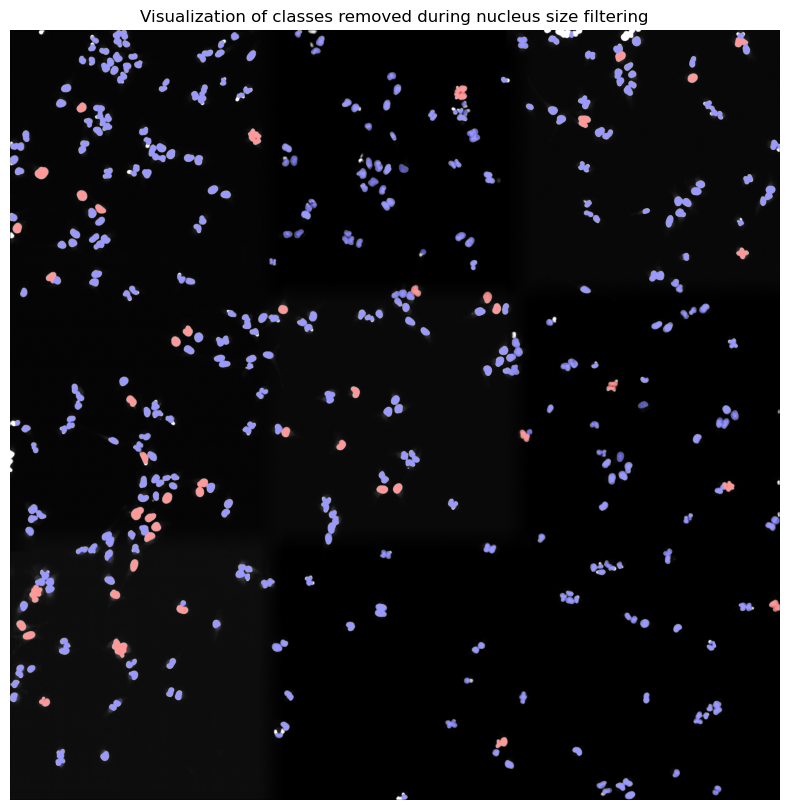
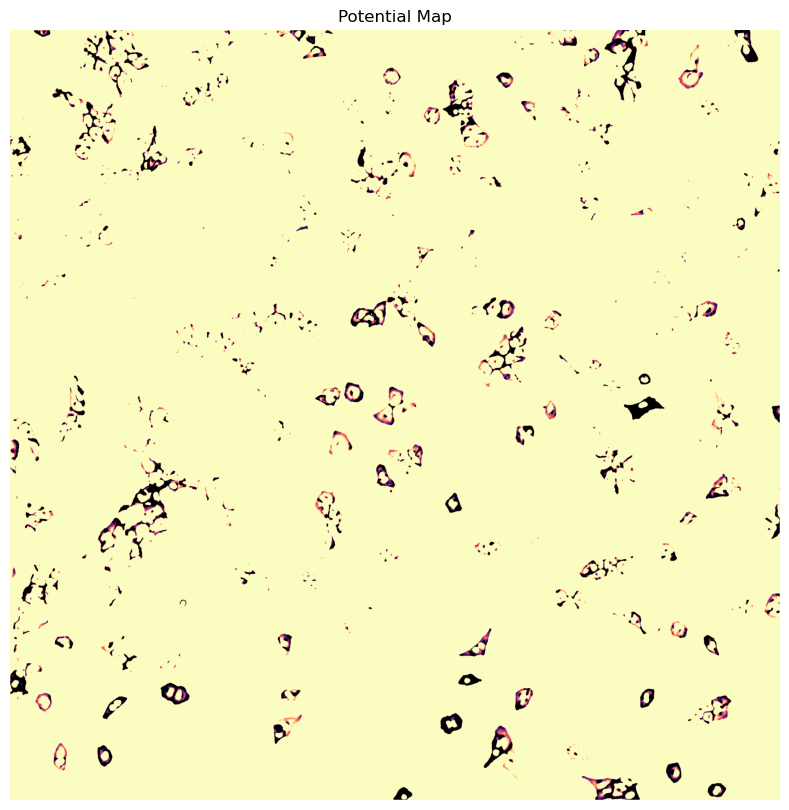
[10/04/2025 21:48:27] Removed 78 nuclei as they fell outside of the threshold range [200, 6000].
[10/04/2025 21:48:28] Total time to perform cytosol size filtering: 3.9210362434387207 seconds
[10/04/2025 21:48:29] Segmentation seg_all_nucleus written to sdata object.
[10/04/2025 21:48:31] Points centers_seg_all_nucleus written to sdata object.
[10/04/2025 21:48:31] Segmentation seg_all_cytosol written to sdata object.
[10/04/2025 21:48:31] Points centers_seg_all_cytosol written to sdata object.
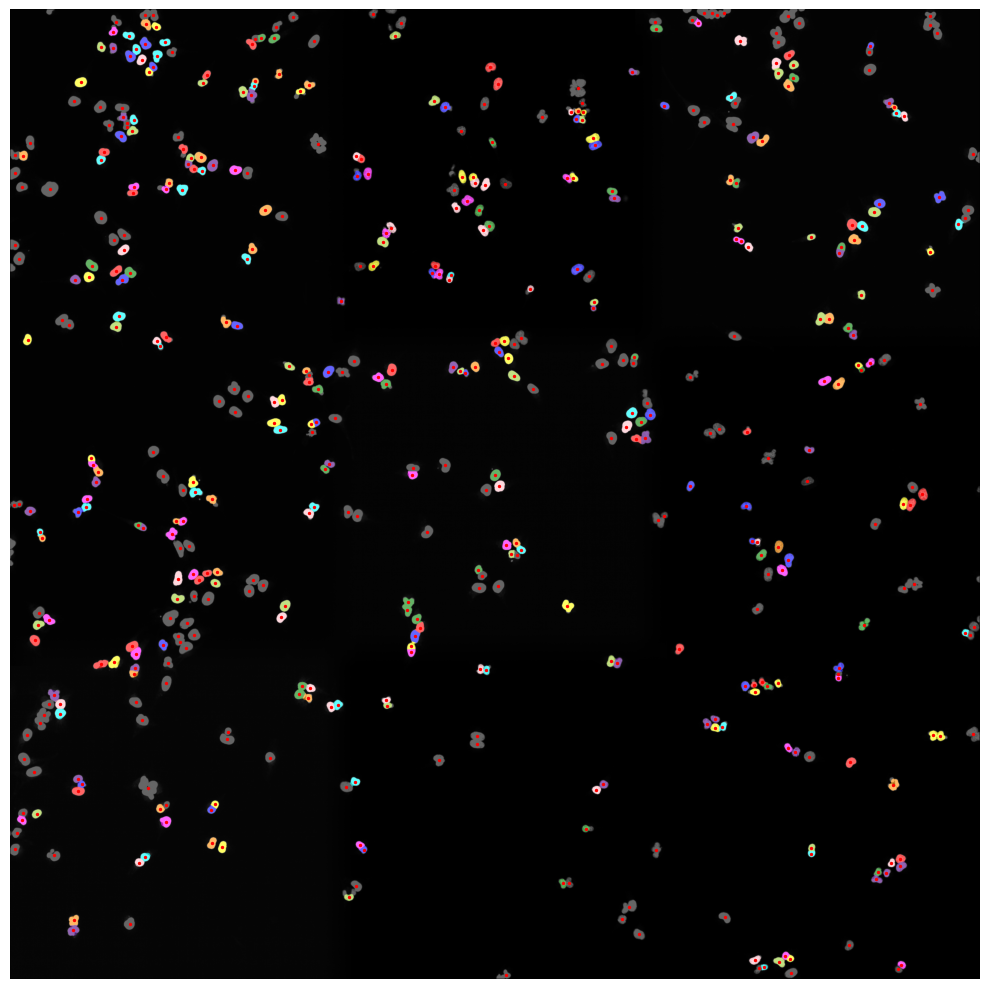
Inspecting Segmentation Results#
Depending on the segmentation method chosen, scPortrait will generate either a single segmentation mask consisting of nuclear or cytosolic segmentation, or both a nuclear and a cytosolic segmentation mask. The resulting masks are stored in the underlying spatialdata object. The labels are annotated with either cytosol or nucleus to indicate which cellular structure has been annotaed.
You can access the spatialdata object with the shortcut project.sdata. Please note that this is only a copy of the object. In case you make any changes (e.g. adding a new element, etc.) you will manually need to write these changes back to disk.
In our case seg_all_cytosol and seg_all_nucleus have been added to our spatialdata object.
[11]:
project.sdata
[11]:
SpatialData object, with associated Zarr store: /Users/sophia/Documents/GitHub/scPortrait/examples/notebooks/example_projects/example_1/project_walkthrough/scportrait.sdata
├── Images
│ └── 'input_image': DataTree[cyx] (3, 3040, 3038), (3, 1520, 1519), (3, 380, 379), (3, 47, 47)
├── Labels
│ ├── 'seg_all_cytosol': DataTree[yx] (3040, 3038), (1520, 1519), (380, 379), (47, 47)
│ └── 'seg_all_nucleus': DataTree[yx] (3040, 3038), (1520, 1519), (380, 379), (47, 47)
└── Points
├── 'centers_seg_all_cytosol': DataFrame with shape: (<Delayed>, 2) (2D points)
└── 'centers_seg_all_nucleus': DataFrame with shape: (<Delayed>, 2) (2D points)
with coordinate systems:
▸ 'global', with elements:
input_image (Images), seg_all_cytosol (Labels), seg_all_nucleus (Labels), centers_seg_all_cytosol (Points), centers_seg_all_nucleus (Points)
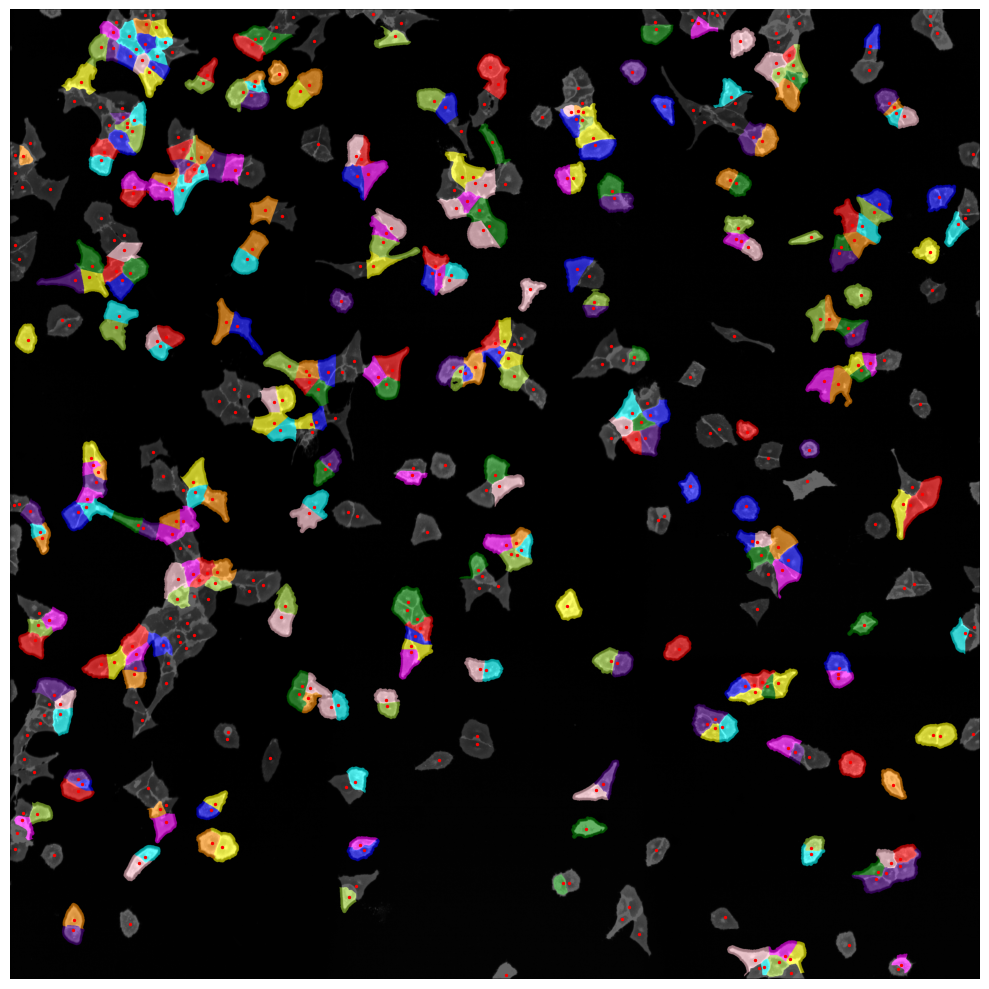
You can also easily visualize the segmentation results using the plot_segmentation_masks method.
[12]:
project.plot_segmentation_masks()
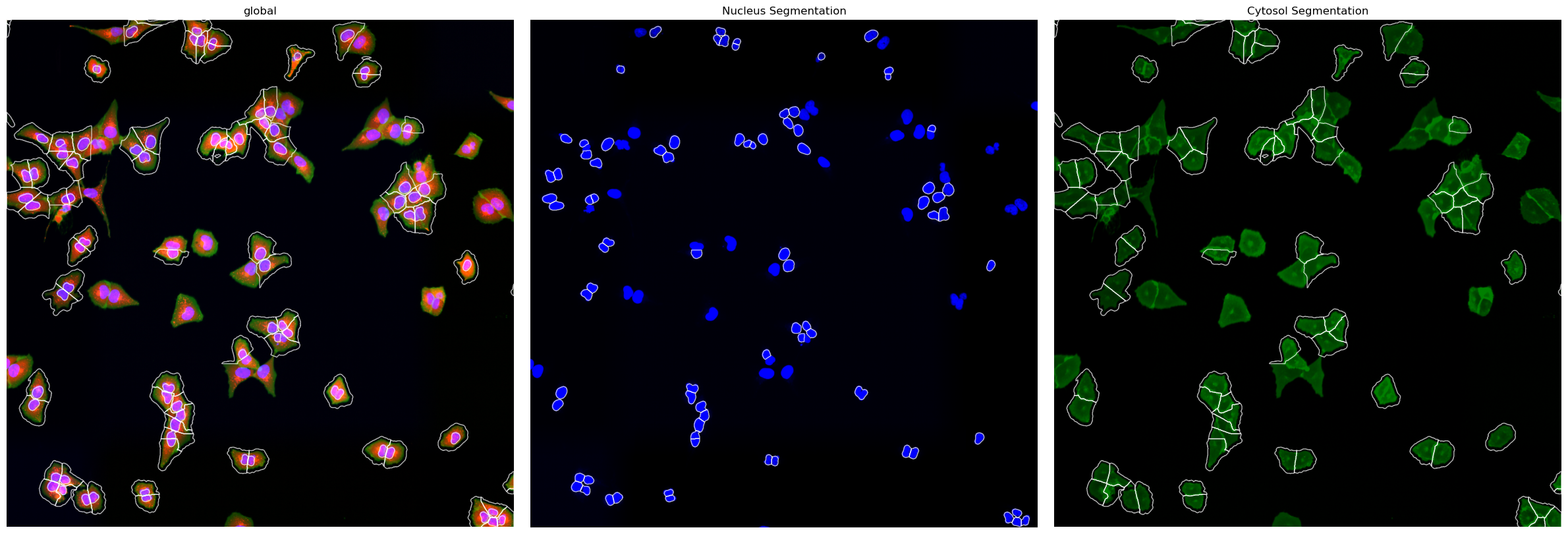
In general the resulting segmentation masks consist of arrays where each pixel is assigned either to background (0) or to a specific cell (cellid: whole number). If we zoom in on the corner of the segmentation mask of a nucleus the numpy array would look like this:
[13]:
plt.imshow(project.sdata.labels["seg_all_nucleus"].scale0.image[230:250, 945:955])
plt.axis("off")
print(project.sdata.labels["seg_all_nucleus"].scale0.image[230:250, 945:955].data.compute())
[[395 395 395 395 395 395 0 0 0 0]
[395 395 395 395 395 395 395 395 0 0]
[395 395 395 395 395 395 395 395 395 0]
[395 395 395 395 395 395 395 395 395 0]
[395 395 395 395 395 395 395 395 395 395]
[395 395 395 395 395 395 395 395 395 395]
[395 395 395 395 395 395 395 395 395 395]
[395 395 395 395 395 395 395 395 395 395]
[395 395 395 395 395 395 395 395 395 395]
[395 395 395 395 395 395 395 395 395 395]
[395 395 395 395 395 395 395 395 395 395]
[395 395 395 395 395 395 395 395 395 0]
[395 395 395 395 395 395 395 395 395 0]
[395 395 395 395 395 395 395 395 0 0]
[395 395 395 395 395 395 0 0 0 0]
[395 395 395 395 0 0 0 0 0 0]
[395 395 0 0 0 0 0 0 0 0]
[395 0 0 0 0 0 0 0 0 0]
[ 0 0 0 0 0 0 0 0 0 0]
[ 0 0 0 0 0 0 0 0 0 0]]
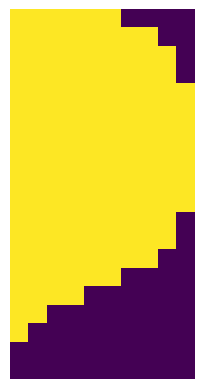
So the mask we are looking at is of the cell with the ID 395. And for each pixel at this location we know if it belongs to the cell or not.
Extracting single cell images#
Once we have generated a segmentation mask, the next step is to extract single-cell images of segmented cells in the dataset. To do this we will use the HDF5CellExtraction method.
The parameters with which HDF5CellExtraction will run are again specified in the config.yml file.
[14]:
display(Markdown(f"```yaml\n{yaml.dump(project.extraction_f.config, default_flow_style=False)}\n```"))
cache: .
compression: true
image_size: 128
threads: 80
[15]:
project.extract()
[10/04/2025 21:48:35] Initialized temporary directory at /var/folders/35/p4c58_4n3bb0bxnzgns1t7kh0000gn/T/./HDF5CellExtraction_3t9u1sp0 for HDF5CellExtraction
[10/04/2025 21:48:35] Created new directory for extraction results: /Users/sophia/Documents/GitHub/scPortrait/examples/notebooks/example_projects/example_1/project_walkthrough/extraction/data
[10/04/2025 21:48:35] Setup output folder at /Users/sophia/Documents/GitHub/scPortrait/examples/notebooks/example_projects/example_1/project_walkthrough/extraction/data
[10/04/2025 21:48:35] Found 2 segmentation masks for the given key in the sdata object. Will be extracting single-cell images based on these masks: ['seg_all_nucleus', 'seg_all_cytosol']
[10/04/2025 21:48:35] Using seg_all_nucleus as the main segmentation mask to determine cell centers.
[10/04/2025 21:48:36] A total of 14 cells were too close to the image border to be extracted. Their cell_ids were saved to file /Users/sophia/Documents/GitHub/scPortrait/examples/notebooks/example_projects/example_1/project_walkthrough/extraction/removed_classes.csv.
[10/04/2025 21:48:36] Container for single-cell data created.
[10/04/2025 21:48:36] Extraction Details:
[10/04/2025 21:48:36] --------------------------------
[10/04/2025 21:48:36] Number of input image channels: 3
[10/04/2025 21:48:36] Number of segmentation masks used during extraction: 2
[10/04/2025 21:48:36] Number of generated output images per cell: 5
[10/04/2025 21:48:36] Number of unique cells to extract: 314
[10/04/2025 21:48:36] Extracted Image Dimensions: 128 x 128
[10/04/2025 21:48:36] Normalization of extracted images: True
[10/04/2025 21:48:36] Percentile normalization range for single-cell images: [0.01, 0.99]
[10/04/2025 21:48:36] Starting single-cell image extraction of 314 cells...
[10/04/2025 21:48:36] Loading input images to memory mapped arrays...
[10/04/2025 21:48:36] Finished transferring data to memory mapped arrays. Time taken: 0.41 seconds.
[10/04/2025 21:48:36] Using batch size of 100 for multiprocessing.
[10/04/2025 21:48:36] Running in multiprocessing mode with 4 threads.
[10/04/2025 21:48:37] Finished extraction in 1.29 seconds (243.16 cells / second)
[10/04/2025 21:48:37] Benchmarking times saved to file.
[10/04/2025 21:48:37] Cleaned up temporary directory at <TemporaryDirectory '/var/folders/35/p4c58_4n3bb0bxnzgns1t7kh0000gn/T/./HDF5CellExtraction_3t9u1sp0'>
Extracted single cell images#
The extracted single-cell images are written to a standardized file formatsingle_cells.h5sc located under extraction\data within the project folder. This data format is built around AnnData. You can easily read the data into an AnnData object allowing you to work with the annotations while keeping the actual single-cell images in memory using the read_h5sc function provided with scportrait.
[16]:
h5sc = scportrait.io.read_h5sc(project.extraction_f.extraction_file)
h5sc
[16]:
AnnData object with n_obs × n_vars = 314 × 5
obs: 'scportrait_cell_id'
var: 'channels', 'channel_mapping'
uns: 'single_cell_images'
obsm: 'single_cell_images'
Alternatively, each project provides a shortcut to its generated h5sc dataset if available.
[17]:
project.h5sc
[17]:
AnnData object with n_obs × n_vars = 314 × 5
obs: 'scportrait_cell_id'
var: 'channels', 'channel_mapping'
uns: 'single_cell_images'
obsm: 'single_cell_images'
Within this format all single-cell images are stored in obsm['single_cell_images'] where each row is a cell and each column is a channel. Additional metadata about how the single-cell images are normalized, which channel is located at which position or how many cells can be found in the dataset is stored in uns['single_cell_images'].
[18]:
display("Single cell image dataset:", h5sc.obsm["single_cell_images"])
display("Metadata:", h5sc.uns["single_cell_images"])
'Single cell image dataset:'
<HDF5 dataset "single_cell_images": shape (314, 5, 128, 128), type "<f2">
'Metadata:'
{'channel_mapping': array(['mask', 'mask', 'image_channel', 'image_channel', 'image_channel'],
dtype=object),
'channel_names': array(['seg_all_nucleus', 'seg_all_cytosol', 'Nucleus', 'Cytosol Marker',
'LC3B'], dtype=object),
'compression': 'lzf',
'image_size': 128,
'n_cells': 314,
'n_channels': 5,
'n_image_channels': 3,
'n_masks': 2,
'normalization': True,
'normalization_range_lower': 0.01,
'normalization_range_upper': 0.99}
Each image is mapped to a unique cell_id letting you trace cells back to their original location within the image. scportrait_cell_ids are stored in the obs slot along with any other annotation. Lets look at an example.
[19]:
h5sc.obs
[19]:
| scportrait_cell_id | |
|---|---|
| 0 | 12 |
| 1 | 13 |
| 2 | 15 |
| 3 | 18 |
| 4 | 33 |
| ... | ... |
| 309 | 491 |
| 310 | 493 |
| 311 | 494 |
| 312 | 495 |
| 313 | 496 |
314 rows × 1 columns
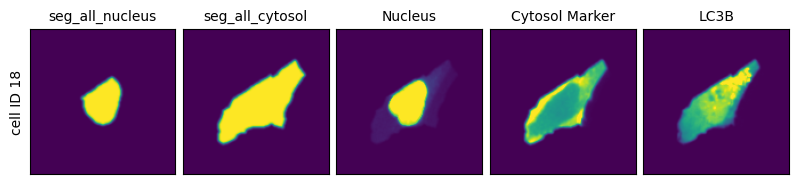
If we now get the image of the cell with the id 18 we can see that it consists of a stack of 5 different views. First the two masks (nucleus and cytosol) followed by the cropped view of the three different channels in our input image.
[20]:
cell_id = 18
image_of_cell = scportrait.tl.h5sc.get_image_with_cellid(h5sc, cell_id)
print(image_of_cell.shape)
fig, axs = plt.subplots(1, 1, figsize=(2 * 5, 2))
scportrait.pl.h5sc._plot_image_grid(ax=axs, images=image_of_cell, nrows=1, ncols=5)
(5, 128, 128)
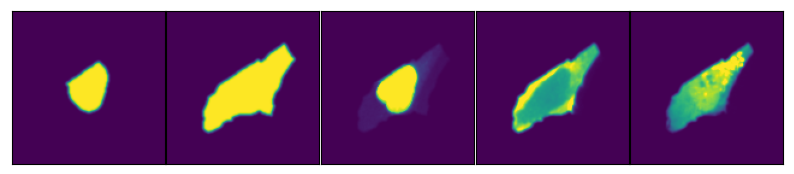
To make visualization easier scPortrait provides some plotting functions which will automatically generate these types of plots for you.
[21]:
scportrait.pl.cell_grid(h5sc, cell_ids=cell_id)
You can also visualize randomly selected cells or also only a single image channel.
[22]:
project.plot_single_cell_images()
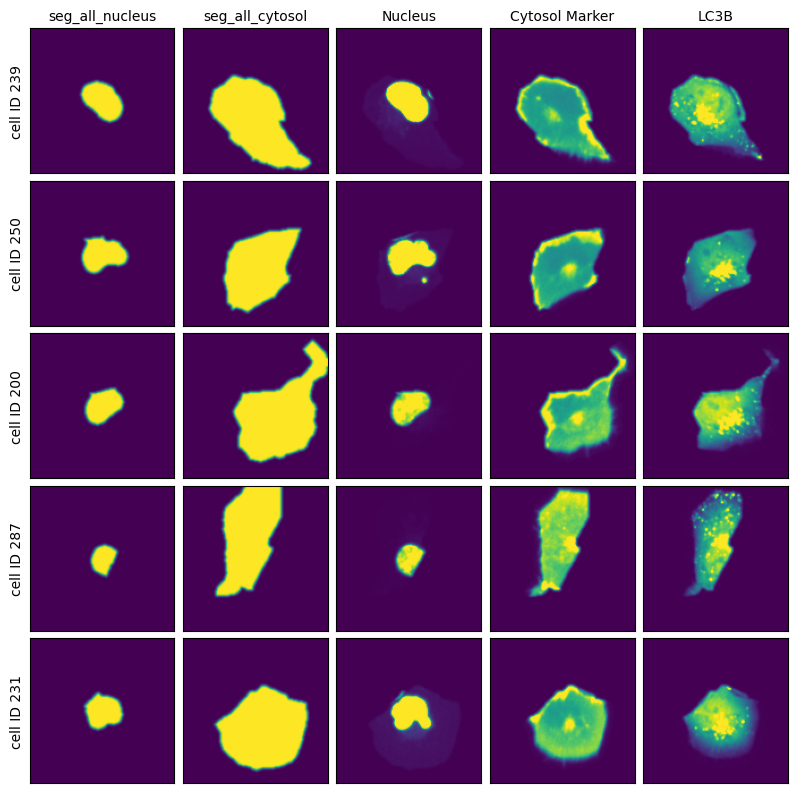
[23]:
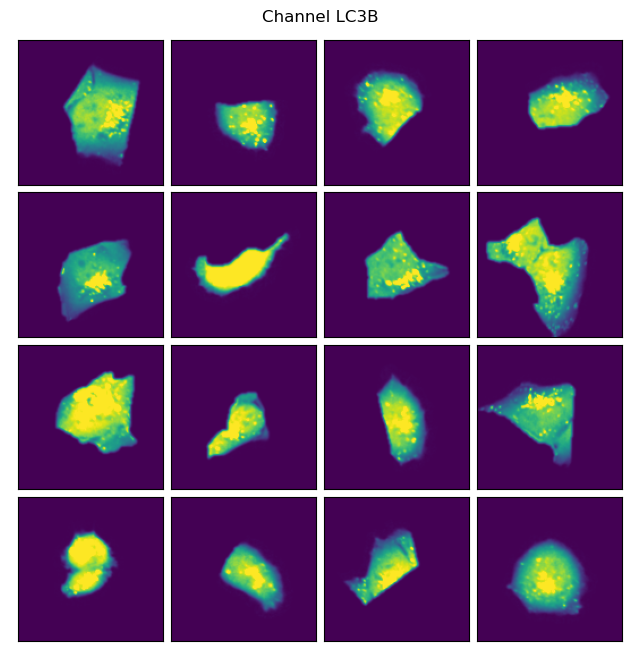
project.plot_single_cell_images(select_channel="LC3B")
Classification of extracted single cells#
Next we can apply a pretained model to classify our cells within the scPortrait project.
Within the config.yml we specify which model should be used for inference and we can give it a name.
MLClusterClassifier:
channel_selection: 4
batch_size: 900
dataloader_worker_number: 10
network: "autophagy_classifier2"
classifier_architecture: "VGG2_old"
label: "Autophagy_15h_classifier2.1"
inference_device: "cpu"
[24]:
display(Markdown(f"```yaml\n{yaml.dump(project.featurization_f.config, default_flow_style=False)}\n```"))
batch_size: 900
cache: /Users/sophia/Documents/GitHub/scPortrait/examples/notebooks/tutorials
channel_selection: 4
dataloader_worker_number: 10
encoders:
- forward
inference_device: cpu
label: Autophagy_15h_classifier2_1
network: autophagy_classifier
Here e.g. we are using a pretrained model included within the scPortrait library autophagy_classifier2.1 and are naming the results from this model Autophagy_15h_classifier2.1.
Model overview:
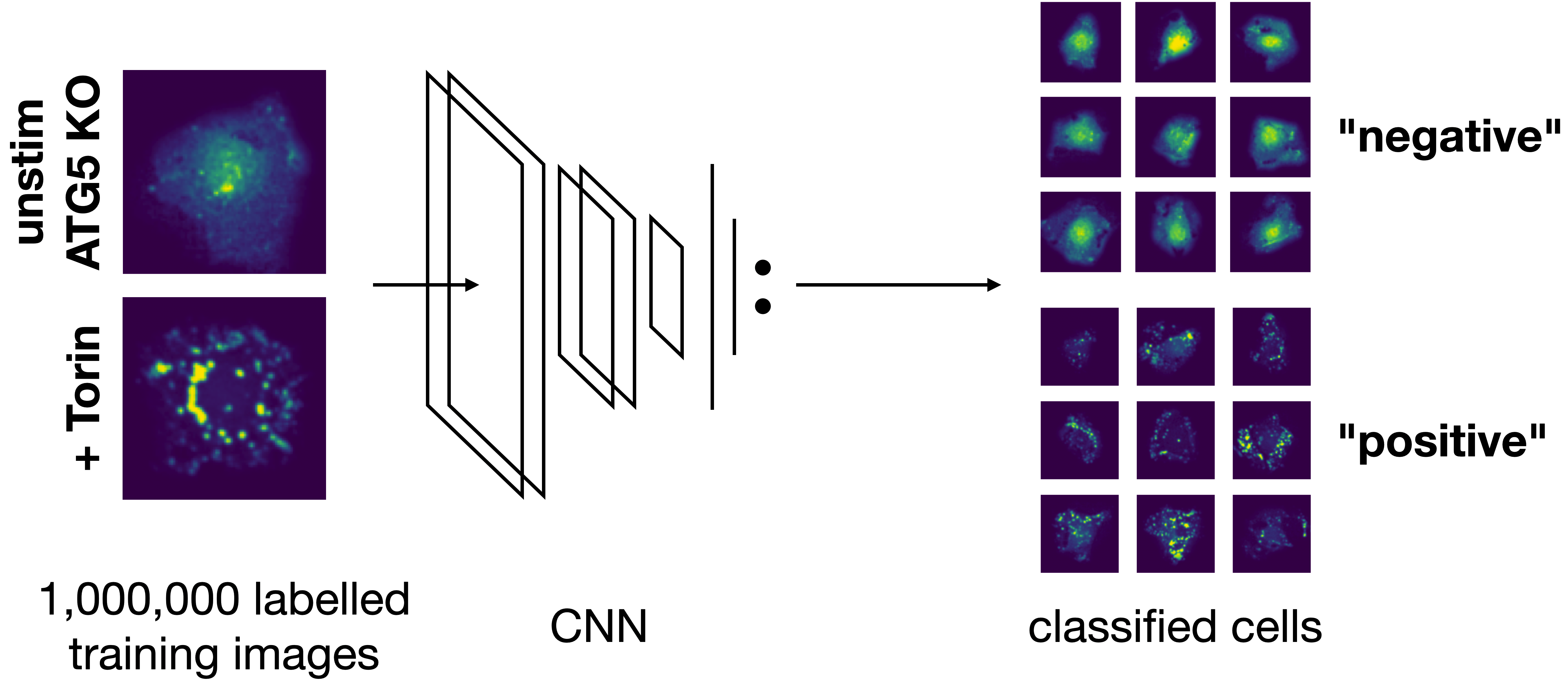
The inference results will be written to a new folder generated under featurization with this name.
If we want to use a model we trained ourselves that is not yet included within the scPortrait library we can simply replace the network name in the config with the path to the checkpoint file generated by pytorch.
[26]:
project.featurize(overwrite = True)
Lightning automatically upgraded your loaded checkpoint from v1.5.5 to v2.5.1. To apply the upgrade to your files permanently, run `python -m pytorch_lightning.utilities.upgrade_checkpoint ../../../src/scportrait/scportrait_data/vgg_autophagy_classifier/VGG2_autophagy_classifier2.1.cpkt`
Using extraction directory: /Users/sophia/Documents/GitHub/scPortrait/examples/notebooks/example_projects/example_1/project_walkthrough/extraction/data/single_cells.h5sc
[10/04/2025 21:49:50] Initialized temporary directory at /Users/sophia/Documents/GitHub/scPortrait/examples/notebooks/tutorials/MLClusterClassifier_z1i4xlhr for MLClusterClassifier
[10/04/2025 21:49:50] Started MLClusterClassifier classification.
[10/04/2025 21:49:50] Overwrite flag is set, deleting existing directory for featurization results.
[10/04/2025 21:49:50] Created new directory for featurization results: /Users/sophia/Documents/GitHub/scPortrait/examples/notebooks/example_projects/example_1/project_walkthrough/featurization/complete_Autophagy_15h_classifier2_1
[10/04/2025 21:49:50] CPU specified in config file but MPS available on system. Consider changing the device for the next run.
[10/04/2025 21:49:50] Model class defined as <class 'scportrait.tools.ml.plmodels.MultilabelSupervisedModel'>
[10/04/2025 21:49:50] Initialized temporary directory at /Users/sophia/Documents/GitHub/scPortrait/examples/notebooks/tutorials/MLClusterClassifier_t43le10v for MLClusterClassifier
[10/04/2025 21:49:50] Reading data from path: /Users/sophia/Documents/GitHub/scPortrait/examples/notebooks/example_projects/example_1/project_walkthrough/extraction/data/single_cells.h5sc
[10/04/2025 21:49:50] Expected image size is set to (128, 128). Resizing images to this size.
[10/04/2025 21:49:50] Processing dataset with 314 cells
[10/04/2025 21:49:50] Dataloader generated with a batchsize of 900 and 10 workers. Dataloader contains 1 entries.
[10/04/2025 21:49:50] Starting inference for model encoder forward
[10/04/2025 21:49:50] Started processing of 1 batches.
[10/04/2025 21:49:52] finished processing.
[10/04/2025 21:49:52] Results saved to file: /Users/sophia/Documents/GitHub/scPortrait/examples/notebooks/example_projects/example_1/project_walkthrough/featurization/complete_Autophagy_15h_classifier2_1/inference_forward.csv
[10/04/2025 21:49:52] Table MLClusterClassifier_Autophagy_15h_classifier2_1_forward_nucleus written to sdata object.
[10/04/2025 21:49:53] Table MLClusterClassifier_Autophagy_15h_classifier2_1_forward_cytosol written to sdata object.
[10/04/2025 21:49:53] Results saved to file: /Users/sophia/Documents/GitHub/scPortrait/examples/notebooks/example_projects/example_1/project_walkthrough/featurization/complete_Autophagy_15h_classifier2_1/inference_forward.csv
[10/04/2025 21:49:53] GPU memory before performing cleanup: None
[10/04/2025 21:49:53] GPU memory after performing cleanup: None
[10/04/2025 21:49:53] Cleaned up temporary directory at <TemporaryDirectory '/Users/sophia/Documents/GitHub/scPortrait/examples/notebooks/tutorials/MLClusterClassifier_t43le10v'>
Looking at the generated results#
The results are written to an anndata object which we can access using the sdata handle.
scPortrait writes the softmax results directly to file as ln() for better precision. To transform these numbers back to the range between 0 and 1 we first need to apply the np.exp function.
[27]:
results = project.sdata["MLClusterClassifier_Autophagy_15h_classifier2_1_forward_nucleus"].to_df()
results["scportrait_cell_id"] = project.sdata["MLClusterClassifier_Autophagy_15h_classifier2_1_forward_nucleus"].obs.scportrait_cell_id
results.result_0 = np.exp(results.result_0)
results.result_1 = np.exp(results.result_1)
results
[27]:
| result_0 | result_1 | scportrait_cell_id | |
|---|---|---|---|
| 0 | 0.020050 | 0.979950 | 12 |
| 1 | 0.000517 | 0.999483 | 13 |
| 2 | 0.809495 | 0.190505 | 15 |
| 3 | 0.206104 | 0.793896 | 18 |
| 4 | 0.623184 | 0.376816 | 33 |
| ... | ... | ... | ... |
| 309 | 0.033211 | 0.966789 | 491 |
| 310 | 0.001642 | 0.998358 | 493 |
| 311 | 0.000081 | 0.999919 | 494 |
| 312 | 0.988729 | 0.011271 | 495 |
| 313 | 0.956842 | 0.043158 | 496 |
314 rows × 3 columns
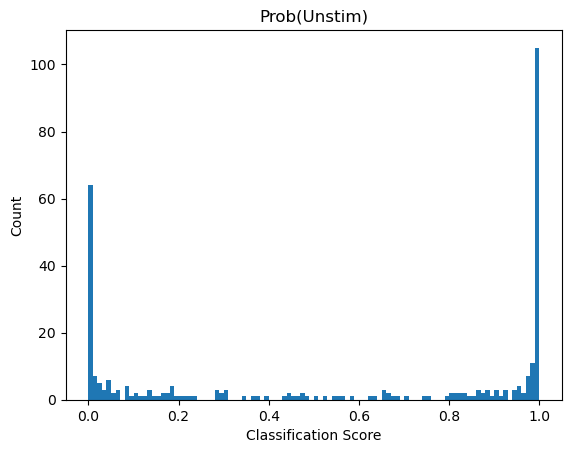
Depending on the model we used what result_0 and result_1 represent will vary. Here we used a binary classification model were class 1 was cells deficient in autophagy. So result_1 indicates the probability score that a cell has the label “autophagy off”. results_0 is simply 1 - result_1
If we look at the distribution in our dataset we can see that almost all cells are classified as “autophagy defective”. Since the input images were from unstimulated wt cells this matches to our expectation.
[28]:
plt.hist(results.result_1, bins=100)
plt.title("Prob(Unstim)")
plt.xlabel("Classification Score")
plt.ylabel("Count");
Lets visualzie the cells with the highest and lowest classification scores.
[29]:
lowest_scores = results.sort_values("result_1").head(16)
highest_scores = results.sort_values("result_1").tail(16)
fig, axs = plt.subplots(1, 2, figsize=(11, 5))
scportrait.pl.cell_grid_single_channel(
project.h5sc,
cell_ids=lowest_scores.scportrait_cell_id.tolist(),
select_channel="LC3B",
ax=axs[0],
show_fig=False,
title="Lowest Scores",
)
scportrait.pl.cell_grid_single_channel(
project.h5sc,
cell_ids=highest_scores.scportrait_cell_id.tolist(),
select_channel="LC3B",
ax=axs[1],
show_fig=False,
title="Highest Scores",
)
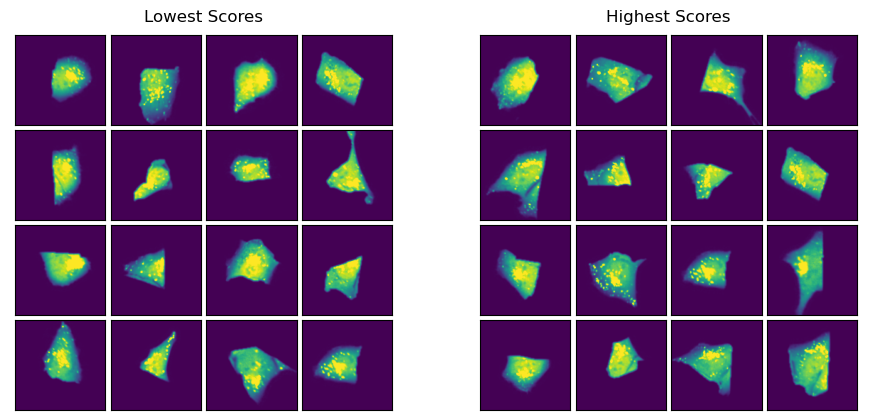
Since these cells are all from the same condition we don’t expect to see big differences between the cells with the lowest and those with the highest score.
Exporting Cutting contours for excision on a Leica LMD7#
scPortrait directly interfaces with our other open-source python library py-lmd to easily select and export cells for excision on a Leica LMD microscope.
Of note: this will require that the cells have been plates on a LMD compatible surface (like a PPS slide). scPortrait can of course also simply be used for data analysis procedures, then ignore this last step.
First we will select the cells we wish to excise based on their classification score. Here we have chosen a threadhold >= 0.99999 for bin1 and a threshold <= 0.9 for bin2.
[30]:
cell_ids_bin1 = results[results.result_1 >= 0.99999].scportrait_cell_id.tolist()
cell_ids_bin2 = results[results.result_1 <= 0.9].scportrait_cell_id.tolist()
print("number of cells to excise:", len(cell_ids_bin1) + len(cell_ids_bin2))
number of cells to excise: 192
The cells can then be allocated into different wells.
[31]:
cells_to_select = [
{"name": "bin1", "classes": list(cell_ids_bin1), "well": "A1"},
{"name": "bin2", "classes": list(cell_ids_bin2), "well": "B1"},
]
In addition to defining which cells we want to excise, we also need to pass the location of the calibration crosses so that we can transfer the image coordinate system into a cutting coordinate system. You can read up on this here.
To obtain the coordinates of your reference points simply open your stitched image in e.g. FIJI and navigate to the location of your reference points. Then write out the coordinates for each point.
[32]:
marker_0 = (0, 0)
marker_1 = (2000, 0)
marker_2 = (0, 2000)
calibration_marker = np.array([marker_0, marker_1, marker_2])
As with the previous methods, additional parameters can be passed to the selection function via the config.yml file which adapts the behaviour of how cutting shapes are generated. You can read up more on this here.
[33]:
display(Markdown(f"```yaml\n{yaml.dump(project.selection_f.config, default_flow_style=False)}\n```"))
cache: .
greedy_k: 15
hilbert_p: 7
orientation_transform:
- - 0
- -1
- - 1
- 0
path_optimization: hilbert
poly_compression_factor: 30
processes_cell_sets: 2
segmentation_channel: seg_all_nucleus
shape_dilation: 16
smoothing_filter_size: 25
threads: 20
[34]:
project.select(cells_to_select, calibration_marker)
[10/04/2025 21:50:09] Initialized temporary directory at /var/folders/35/p4c58_4n3bb0bxnzgns1t7kh0000gn/T/./LMDSelection_q_gqg0vi for LMDSelection
[10/04/2025 21:50:09] Selection process started.
[10/04/2025 21:50:17] Temporary directory not found, skipping cleanup
[10/04/2025 21:50:17] Temporary directory not found, skipping cleanup
[10/04/2025 21:50:18] Coordinate lookup index calculation took 8.743029665987706 seconds.
No configuration for shape_erosion found, parameter will be set to 0
No configuration for binary_smoothing found, parameter will be set to 3
No configuration for convolution_smoothing found, parameter will be set to 15
No configuration for rdp_epsilon found, parameter will be set to 0.1
No configuration for xml_decimal_transform found, parameter will be set to 100
No configuration for distance_heuristic found, parameter will be set to 300
No configuration for join_intersecting found, parameter will be set to True
Path optimizer used for XML generation: hilbert
cell set 0 passed sanity check
cell set 1 passed sanity check
Loading coordinates from external source
Processing cell sets in parallel
Convert label format into coordinate format
Conversion finished, performing sanity check.
Intersecting Shapes will be merged into a single shape.
Convert label format into coordinate format
Conversion finished, performing sanity check.
Intersecting Shapes will be merged into a single shape.
dilating shapes: 100%|██████████| 17/17 [00:04<00:00, 3.91it/s]
dilating shapes: 100%|██████████| 175/175 [00:03<00:00, 45.19it/s]
creating shapes: 0%| | 0/16 [00:00<?, ?it/s]
0 shapes that were intersecting were found and merged.
0 shapes that were intersecting were found and merged.
creating shapes: 100%|██████████| 16/16 [00:03<00:00, 4.18it/s]
creating shapes: 100%|██████████| 80/80 [00:04<00:00, 17.41it/s]
calculating polygons: 100%|██████████| 16/16 [00:04<00:00, 3.48it/s]
Current path length: 22,306.05 units
Optimized path length: 9,837.50 units
Optimization factor: 2.3x
calculating polygons: 1%|▏ | 1/80 [00:03<04:03, 3.08s/it]
Plotting shapes in debug mode is not supported in multi-threading mode.
Saving plots to disk instead.
calculating polygons: 100%|██████████| 80/80 [00:04<00:00, 19.36it/s]
Current path length: 109,484.75 units
Optimized path length: 24,906.79 units
Optimization factor: 4.4x
Plotting shapes in debug mode is not supported in multi-threading mode.
Saving plots to disk instead.
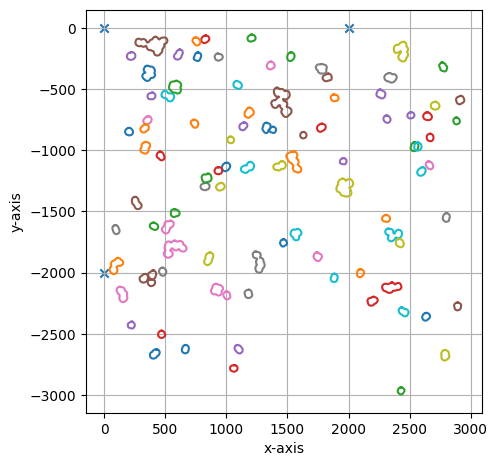
===== Collection Stats =====
Number of shapes: 96
Number of vertices: 8,036
============================
Mean vertices: 84
Min vertices: 53
5% percentile vertices: 59
Median vertices: 68
95% percentile vertices: 160
Max vertices: 287
[0 0]
[ 0 -200000]
[200000 0]
[10/04/2025 21:50:35] Saved output at /Users/sophia/Documents/GitHub/scPortrait/examples/notebooks/example_projects/example_1/project_walkthrough/selection/bin1_bin2.xml
[10/04/2025 21:50:35] Cleaned up temporary directory at <TemporaryDirectory '/var/folders/35/p4c58_4n3bb0bxnzgns1t7kh0000gn/T/./LMDSelection_q_gqg0vi'>
[ ]: